Small-molecule-induced epigenetic rejuvenation promotes SREBP condensation and overcomes barriers to CNS myelin regeneration
- PMID: 38701782
- PMCID: PMC11812128
- DOI: 10.1016/j.cell.2024.04.005
Small-molecule-induced epigenetic rejuvenation promotes SREBP condensation and overcomes barriers to CNS myelin regeneration
Abstract
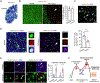
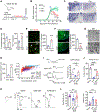
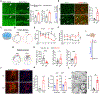
Remyelination failure in diseases like multiple sclerosis (MS) was thought to involve suppressed maturation of oligodendrocyte precursors; however, oligodendrocytes are present in MS lesions yet lack myelin production. We found that oligodendrocytes in the lesions are epigenetically silenced. Developing a transgenic reporter labeling differentiated oligodendrocytes for phenotypic screening, we identified a small-molecule epigenetic-silencing-inhibitor (ESI1) that enhances myelin production and ensheathment. ESI1 promotes remyelination in animal models of demyelination and enables de novo myelinogenesis on regenerated CNS axons. ESI1 treatment lengthened myelin sheaths in human iPSC-derived organoids and augmented (re)myelination in aged mice while reversing age-related cognitive decline. Multi-omics revealed that ESI1 induces an active chromatin landscape that activates myelinogenic pathways and reprograms metabolism. Notably, ESI1 triggered nuclear condensate formation of master lipid-metabolic regulators SREBP1/2, concentrating transcriptional co-activators to drive lipid/cholesterol biosynthesis. Our study highlights the potential of targeting epigenetic silencing to enable CNS myelin regeneration in demyelinating diseases and aging.
Keywords: Epigenetic silencing; HDAC3 Inhibition; SREBP condensation; aging; chromatin remodeling; lipid/cholesterol biosynthesis; multiple sclerosis; myelin regeneration; oligodendrocyte; small molecule.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Blakemore WF, and Franklin RJ (2008). Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol 318, 193–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

