CHMP2A regulates broad immune cell-mediated antitumor activity in an immunocompetent in vivo head and neck squamous cell carcinoma model
- PMID: 38702144
- PMCID: PMC11086353
- DOI: 10.1136/jitc-2023-007187
CHMP2A regulates broad immune cell-mediated antitumor activity in an immunocompetent in vivo head and neck squamous cell carcinoma model
Abstract
Background: Natural killer (NK) cells are key effector cells of antitumor immunity. However, tumors can acquire resistance programs to escape NK cell-mediated immunosurveillance. Identifying mechanisms that mediate this resistance enables us to define approaches to improve immune-mediate antitumor activity. In previous studies from our group, a genome-wide CRISPR-Cas9 screen identified Charged Multivesicular Body Protein 2A (CHMP2A) as a novel mechanism that mediates tumor intrinsic resistance to NK cell activity.
Methods: Here, we use an immunocompetent mouse model to demonstrate that CHMP2A serves as a targetable regulator of not only NK cell-mediated immunity but also other immune cell populations. Using the recently characterized murine 4MOSC model system, a syngeneic, tobacco-signature murine head and neck squamous cell carcinoma model, we deleted mCHMP2A using CRISPR/Cas9-mediated knock-out (KO), following orthotopic transplantation into immunocompetent hosts.
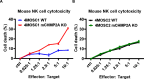
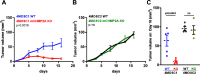
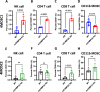
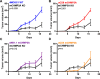
Results: We found that mCHMP2A KO in 4MOSC1 cells leads to more potent NK-mediated tumor cell killing in vitro in these tumor cells. Moreover, following orthotopic transplantation, KO of mCHMP2A in 4MOSC1 cells, but not the more immune-resistant 4MOSC2 cells enables both T cells and NK cells to better mediate antitumor activity compared with wild type (WT) tumors. However, there was no difference in tumor development between WT and mCHMP2A KO 4MOSC1 or 4MOSC2 tumors when implanted in immunodeficient mice. Mechanistically, we find that mCHMP2A KO 4MOSC1 tumors transplanted into the immunocompetent mice had significantly increased CD4+T cells, CD8+T cells. NK cell, as well as fewer myeloid-derived suppressor cells (MDSC).
Conclusions: Together, these studies demonstrate that CHMP2A is a targetable inhibitor of cellular antitumor immunity.
Keywords: Immunity, Innate; Immunotherapy; Immunotherapy, Adoptive; Killer Cells, Natural; Myeloid-Derived Suppressor Cells.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DSK is a co-founder and advisor to Shoreline Biosciences and has an equity interest in the company. DSK also consults for Qihan Biotech and VisiCELL Medical for which he receives income and/or equity. Studies in this work are not related to the work of those companies. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. JSG reports consulting fees from Domain Pharmaceuticals, Pangea Therapeutics, and io9, and is the founder of Kadima Pharmaceuticals.
Figures


Similar articles
-
CHMP2A regulates tumor sensitivity to natural killer cell-mediated cytotoxicity.Nat Commun. 2022 Apr 7;13(1):1899. doi: 10.1038/s41467-022-29469-0. Nat Commun. 2022. PMID: 35393416 Free PMC article.
-
Chimeric antigen receptor engineered NK cellular immunotherapy overcomes the selection of T-cell escape variant cancer cells.J Immunother Cancer. 2021 Mar;9(3):e002128. doi: 10.1136/jitc-2020-002128. J Immunother Cancer. 2021. PMID: 33741731 Free PMC article.
-
Differences in TCR repertoire and T cell activation underlie the divergent outcomes of antitumor immune responses in tumor-eradicating versus tumor-progressing hosts.J Immunother Cancer. 2021 Jan;9(1):e001615. doi: 10.1136/jitc-2020-001615. J Immunother Cancer. 2021. PMID: 33414263 Free PMC article.
-
Landscape of natural killer cell activity in head and neck squamous cell carcinoma.J Immunother Cancer. 2020 Dec;8(2):e001523. doi: 10.1136/jitc-2020-001523. J Immunother Cancer. 2020. PMID: 33428584 Free PMC article. Review.
-
Tumor microenvironmental modification by the current target therapy for head and neck squamous cell carcinoma.J Exp Clin Cancer Res. 2023 May 5;42(1):114. doi: 10.1186/s13046-023-02691-4. J Exp Clin Cancer Res. 2023. PMID: 37143088 Free PMC article. Review.
Cited by
-
VPS25 Promotes an Immunosuppressive Microenvironment in Head and Neck Squamous Cell Carcinoma.Biomolecules. 2025 Feb 22;15(3):323. doi: 10.3390/biom15030323. Biomolecules. 2025. PMID: 40149859 Free PMC article.
-
NK cell based immunotherapy against oral squamous cell carcinoma.Front Immunol. 2024 Aug 13;15:1440764. doi: 10.3389/fimmu.2024.1440764. eCollection 2024. Front Immunol. 2024. PMID: 39192980 Free PMC article. Review.
-
A strategy for synergistic enhancement of immune circulation in head and neck squamous cell carcinoma by novel nucleic acid drug therapy and immunotherapy.J Transl Med. 2025 Mar 20;23(1):354. doi: 10.1186/s12967-025-06344-2. J Transl Med. 2025. PMID: 40114181 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
