Combining toll-like receptor agonists with immune checkpoint blockade affects antitumor vaccine efficacy
- PMID: 38702146
- PMCID: PMC11086196
- DOI: 10.1136/jitc-2024-008799
Combining toll-like receptor agonists with immune checkpoint blockade affects antitumor vaccine efficacy
Abstract
Background: T cell checkpoint receptors are expressed when T cells are activated, and modulation of the expression or signaling of these receptors can alter the function of T cells and their antitumor efficacy. We previously found that T cells activated with cognate antigen had increases in the expression of PD-1, and this was attenuated in the presence of multiple toll-like receptor (TLR) agonists, notably TLR3 plus TLR9. In the current report, we sought to investigate whether combining TLR agonists with immune checkpoint blockade can further augment vaccine-mediated T cell antitumor immunity in murine tumor models.
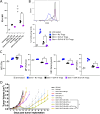
Methods: TLR agonists (TLR3 plus TLR9) and immune checkpoint inhibitors (antibodies targeting PD-1, CTLA-4, LAG-3, TIM-3 or VISTA) were combined and delivered with vaccines or vaccine-activated CD8+T cells to E.G7-OVA or MyC-CaP tumor-bearing mice. Tumors were assessed for growth and then collected and analyzed by flow cytometry.
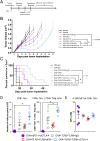
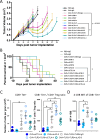
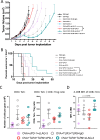
Results: Immunization of E.G7-OVA tumor-bearing mice with SIINFEKL peptide vaccine, coadministered with TLR agonists and αCTLA-4, demonstrated greater antitumor efficacy than immunization with TLR agonists or αCTLA-4 alone. Conversely, the antitumor efficacy was abrogated when vaccine and TLR agonists were combined with αPD-1. TLR agonists suppressed PD-1 expression on regulatory T cells (Tregs) and activated this population. Depletion of Tregs in tumor-bearing mice led to greater antitumor efficacy of this combination therapy, even in the presence of αPD-1. Combining vaccination with TLR agonists and αCTLA-4 or αLAG-3 showed greater antitumor than with combinations with αTIM-3 or αVISTA.
Conclusion: The combination of TLR agonists and αCTLA-4 or αLAG-3 can further improve the efficacy of a cancer vaccine, an effect not observed using αPD-1 due to activation of Tregs when αPD-1 was combined with TLR3 and TLR9 agonists. These data suggest that optimal combinations of TLR agonists and immune checkpoint blockade may improve the efficacy of human anticancer vaccines.
Keywords: Immune Checkpoint Inhibitor; Prostate Cancer; T regulatory cell - Treg; Toll-like receptor - TLR; Vaccine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DGM has ownership interest, has received research support and serves as consultant to Madison Vaccines, which has licensed the pTVG-AR vaccine described in this manuscript. The other authors have no relevant potential conflicts of interest.
Figures
References
-
- Tomai MA, Vasilakos JP. TLR agonists as vaccine adjuvants. In: Baschieri S, ed. Innovation in Vaccinology: from design, through to delivery and testing. Dordrecht: Springer Netherlands, 2012: 205–28.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
