Gentamicin loaded niosomes against intracellular uropathogenic Escherichia coli strains
- PMID: 38702355
- PMCID: PMC11068731
- DOI: 10.1038/s41598-024-59144-x
Gentamicin loaded niosomes against intracellular uropathogenic Escherichia coli strains
Abstract
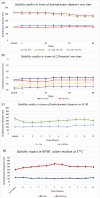
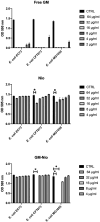
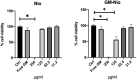
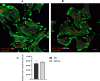
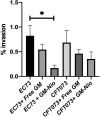
Urinary tract infections (UTIs) are the most common bacterial infections and uropathogenic Escherichia coli (UPEC) is the main etiological agent of UTIs. UPEC can persist in bladder cells protected by immunological defenses and antibiotics and intracellular behavior leads to difficulty in eradicating the infection. The aim of this paper is to design, prepare and characterize surfactant-based nanocarriers (niosomes) able to entrap antimicrobial drug and potentially to delivery and release antibiotics into UPEC-infected cells. In order to validate the proposed drug delivery system, gentamicin, was chosen as "active model drug" due to its poor cellular penetration. The niosomes physical-chemical characterization was performed combining different techniques: Dynamic Light Scattering Fluorescence Spectroscopy, Transmission Electron Microscopy. Empty and loaded niosomes were characterized in terms of size, ζ-potential, bilayer features and stability. Moreover, Gentamicin entrapped amount was evaluated, and the release study was also carried out. In addition, the effect of empty and loaded niosomes was studied on the invasion ability of UPEC strains in T24 bladder cell monolayers by Gentamicin Protection Assay and Confocal Microscopy. The observed decrease in UPEC invasion rate leads us to hypothesize a release of antibiotic from niosomes inside the cells. The optimization of the proposed drug delivery system could represent a promising strategy to significatively enhance the internalization of antimicrobial drugs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

