Generation of 'semi-guided' cortical organoids with complex neural oscillations
- PMID: 38702386
- PMCID: PMC11380594
- DOI: 10.1038/s41596-024-00994-0
Generation of 'semi-guided' cortical organoids with complex neural oscillations
Erratum in
-
Publisher Correction: Generation of 'semi-guided' cortical organoids with complex neural oscillations.Nat Protoc. 2024 Oct 15. doi: 10.1038/s41596-024-01087-8. Online ahead of print. Nat Protoc. 2024. PMID: 39407039 No abstract available.
Abstract
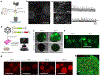
Temporal development of neural electrophysiology follows genetic programming, similar to cellular maturation and organization during development. The emergent properties of this electrophysiological development, namely neural oscillations, can be used to characterize brain development. Recently, we utilized the innate programming encoded in the human genome to generate functionally mature cortical organoids. In brief, stem cells are suspended in culture via continuous shaking and naturally aggregate into embryoid bodies before being exposed to media formulations for neural induction, differentiation and maturation. The specific culture format, media composition and duration of exposure to these media distinguish organoid protocols and determine whether a protocol is guided or unguided toward specific neural fate. The 'semi-guided' protocol presented here has shorter induction and differentiation steps with less-specific patterning molecules than most guided protocols but maintains the use of neurotrophic factors such as brain-derived growth factor and neurotrophin-3, unlike unguided approaches. This approach yields the cell type diversity of unguided approaches while maintaining reproducibility for disease modeling. Importantly, we characterized the electrophysiology of these organoids and found that they recapitulate the maturation of neural oscillations observed in the developing human brain, a feature not shown with other approaches. This protocol represents the potential first steps toward bridging molecular and cellular biology to human cognition, and it has already been used to discover underlying features of human brain development, evolution and neurological conditions. Experienced cell culture technicians can expect the protocol to take 1 month, with extended maturation, electrophysiology recording, and adeno-associated virus transduction procedure options.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
A.R.M. is a cofounder of and has an equity interest in TISMOO, a company dedicated to genetic analysis and brain organoid modeling focusing on therapeutic applications customized for autism spectrum disorder and other neurological disorders with genetic origins. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict-of-interest policies. A.R.M. is an inventor of several patents related to human functional brain organogenesis, including the protocol described here.
Figures





References
-
- Grenier K, Kao J & Diamandis P Three-dimensional modeling of human neurodegeneration: brain organoids coming of age. Mol. Psychiatry 25, 254–274 (2020). - PubMed
-
- Eiraku M et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008). - PubMed
Key references
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
- R01 MH127077/MH/NIMH NIH HHS/United States
- R01 DA056908/DA/NIDA NIH HHS/United States
- R01 MH123828/MH/NIMH NIH HHS/United States
- W81XWH2110306/U.S. Department of Defense (United States Department of Defense)
- R01 ES033636/ES/NIEHS NIH HHS/United States
- R56 MH128365/MH/NIMH NIH HHS/United States
- U19 MH107367/MH/NIMH NIH HHS/United States
- R21 MH128827/MH/NIMH NIH HHS/United States
- DISC2-13515/California Institute for Regenerative Medicine (CIRM)
- R01 NS105969/NS/NINDS NIH HHS/United States
- R01 HD107788/HD/NICHD NIH HHS/United States
- R01 MH100175/MH/NIMH NIH HHS/United States
- R01 HG012351/HG/NHGRI NIH HHS/United States
- R01 NS123642/NS/NINDS NIH HHS/United States
- R01 AG078959/AG/NIA NIH HHS/United States
- R21 HD109616/HD/NICHD NIH HHS/United States
- RF1 AG084030/AG/NIA NIH HHS/United States

