Persistent T cell unresponsiveness associated with chronic visceral leishmaniasis in HIV-coinfected patients
- PMID: 38702419
- PMCID: PMC11068874
- DOI: 10.1038/s42003-024-06225-2
Persistent T cell unresponsiveness associated with chronic visceral leishmaniasis in HIV-coinfected patients
Abstract
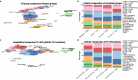
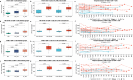
A large proportion of HIV-coinfected visceral leishmaniasis (VL-HIV) patients exhibit chronic disease with frequent VL recurrence. However, knowledge on immunological determinants underlying the disease course is scarce. We longitudinally profiled the circulatory cellular immunity of an Ethiopian HIV cohort that included VL developers. We show that chronic VL-HIV patients exhibit high and persistent levels of TIGIT and PD-1 on CD8+/CD8- T cells, in addition to a lower frequency of IFN-γ+ TIGIT- CD8+/CD8- T cells, suggestive of impaired T cell functionality. At single T cell transcriptome and clonal resolution, the patients show CD4+ T cell anergy, characterised by a lack of T cell activation and lymphoproliferative response. These findings suggest that PD-1 and TIGIT play a pivotal role in VL-HIV chronicity, and may be further explored for patient risk stratification. Our findings provide a strong rationale for adjunctive immunotherapy for the treatment of chronic VL-HIV patients to break the recurrent disease cycle.
© 2024. The Author(s).
Conflict of interest statement
K.L. and P.M. hold shares in ImmuneWatch™, an immunoinformatics company. ImmuneWatch™ had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors declare no conflict of interest.
Figures





Similar articles
-
Insights to the HIV-associated visceral leishmaniasis clinical outcome: lessons learned about immune mediated disorders.Front Immunol. 2025 Mar 12;16:1516176. doi: 10.3389/fimmu.2025.1516176. eCollection 2025. Front Immunol. 2025. PMID: 40145085 Free PMC article. Review.
-
Immunological factors, but not clinical features, predict visceral leishmaniasis relapse in patients co-infected with HIV.Cell Rep Med. 2021 Dec 30;3(1):100487. doi: 10.1016/j.xcrm.2021.100487. eCollection 2022 Jan 18. Cell Rep Med. 2021. PMID: 35106507 Free PMC article.
-
T-cell activation and senescence in asymptomatic HIV/Leishmania infantum co-infection.PLoS Negl Trop Dis. 2025 Mar 17;19(3):e0012848. doi: 10.1371/journal.pntd.0012848. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40096173 Free PMC article.
-
Multifunctional, TNF-α and IFN-γ-Secreting CD4 and CD8 T Cells and CD8High T Cells Are Associated With the Cure of Human Visceral Leishmaniasis.Front Immunol. 2021 Oct 28;12:773983. doi: 10.3389/fimmu.2021.773983. eCollection 2021. Front Immunol. 2021. PMID: 34777391 Free PMC article.
-
The nexus between Leishmania & HIV: Debilitating host immunity and Hastening Comorbid disease burden.Exp Parasitol. 2024 Oct;265:108826. doi: 10.1016/j.exppara.2024.108826. Epub 2024 Aug 13. Exp Parasitol. 2024. PMID: 39147120 Review.
Cited by
-
Prediction of visceral leishmaniasis development in a highly exposed HIV cohort in Ethiopia based on Leishmania infection markers: results from the PreLeisH study.EBioMedicine. 2024 Dec;110:105474. doi: 10.1016/j.ebiom.2024.105474. Epub 2024 Nov 29. EBioMedicine. 2024. PMID: 39612653 Free PMC article.
-
Serum sPD-1 as a marker of T cell exhaustion in ART-naïve, ART-experienced, and intestinal parasite co-infected HIV-positive adults at the university of Gondar comprehensive specialized hospital, Northwest Ethiopia, 2024.BMC Infect Dis. 2025 May 27;25(1):765. doi: 10.1186/s12879-025-11158-0. BMC Infect Dis. 2025. PMID: 40426096 Free PMC article.
-
AI-driven analysis by identifying risk factors of VL relapse in HIV co-infected patients.Sci Rep. 2025 Jul 1;15(1):21067. doi: 10.1038/s41598-025-07406-7. Sci Rep. 2025. PMID: 40596278 Free PMC article.
-
Insights to the HIV-associated visceral leishmaniasis clinical outcome: lessons learned about immune mediated disorders.Front Immunol. 2025 Mar 12;16:1516176. doi: 10.3389/fimmu.2025.1516176. eCollection 2025. Front Immunol. 2025. PMID: 40145085 Free PMC article. Review.
-
Visceral leishmaniasis in heavily pretreated multiple myeloma patients a case series of 17 patients from the Mediterranean Coast of Spain.Ann Med. 2025 Dec;57(1):2514075. doi: 10.1080/07853890.2025.2514075. Epub 2025 Jun 5. Ann Med. 2025. PMID: 40470770 Free PMC article.
References
-
- Burza, S., Croft, S. L. & Boelaert, M. L. Lancet392, 951–970 (2018). - PubMed
-
- WHO. Leishmaniasis, https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (2022).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

