Follicle-stimulating hormone receptor expression in advanced atherosclerotic plaques
- PMID: 38702476
- PMCID: PMC11068877
- DOI: 10.1038/s41598-024-60962-2
Follicle-stimulating hormone receptor expression in advanced atherosclerotic plaques
Abstract
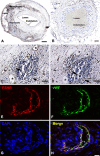
Experimental evidence indicates that follicle-stimulating hormone (FSH), an essential hormone for reproduction, can act directly on endothelial cells inducing atherosclerosis activation and development. However, it remains unknown whether the FSH-receptor (FSHR) is expressed in human atherosclerosis plaques. To demonstrate the FSHR presence, we used immunohistochemical and immunoelectron microscopy involving a specific monoclonal antibody FSHR1A02 that recognizes an epitope present in the FSHR-ectodomain. In all 55 patients with atherosclerotic plaques located in carotid, coronary, femoral arteries, and iliac aneurysm, FSHR was selectively expressed in arterial endothelium covering atherosclerotic plaques and endothelia lining intraplaque neovessels. Lymphatic neovessels were negative for FSHR. M1-macrophages, foam cells, and giant multinucleated cells were also FSHR-positive. FSHR was not detected in normal internal thoracic artery. Immunoelectron microscopy performed in ApoEKO/hFSHRKI mice with atherosclerotic plaques, after injection in vivo with mouse anti-hFSHR monoclonal antibody FSHR1A02 coupled to colloidal gold, showed FSHR presence on the luminal surface of arterial endothelial cells covering atherosclerotic plaques. Therefore, FSHR can bind, internalize, and deliver into the plaque circulating ligands to FSHR-positive cells. In conclusion, we report FSHR expression in endothelial cells, M1-macrophages, M1-derived foam cells, giant multinucleated macrophages, and osteoclasts associated with human atherosclerotic plaques.
Keywords: Atherosclerosis; Atherosclerotic plaque; FSH; FSHR; FSHR1 isoform; FSHR1A02 antibody; Splenic aneurysm.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Exploring the expression and potential function of follicle stimulating hormone receptor in extragonadal cells related to abdominal aortic aneurysm.PLoS One. 2023 May 25;18(5):e0285607. doi: 10.1371/journal.pone.0285607. eCollection 2023. PLoS One. 2023. PMID: 37228156 Free PMC article.
-
Follicular Stimulating Hormone Accelerates Atherogenesis by Increasing Endothelial VCAM-1 Expression.Theranostics. 2017 Oct 17;7(19):4671-4688. doi: 10.7150/thno.21216. eCollection 2017. Theranostics. 2017. PMID: 29187895 Free PMC article.
-
Phage display identification of CD100 in human atherosclerotic plaque macrophages and foam cells.PLoS One. 2013 Sep 30;8(9):e75772. doi: 10.1371/journal.pone.0075772. eCollection 2013. PLoS One. 2013. PMID: 24098722 Free PMC article.
-
Macrophage-mediated proteolytic remodeling of the extracellular matrix in atherosclerosis results in neoepitopes: a potential new class of biochemical markers.Assay Drug Dev Technol. 2010 Oct;8(5):542-52. doi: 10.1089/adt.2009.0258. Epub 2010 Jul 27. Assay Drug Dev Technol. 2010. PMID: 20662734 Review.
-
Follicle-Stimulating Hormone Receptor: Advances and Remaining Challenges.Int Rev Cell Mol Biol. 2018;338:1-58. doi: 10.1016/bs.ircmb.2018.02.001. Epub 2018 Apr 5. Int Rev Cell Mol Biol. 2018. PMID: 29699689 Review.
Cited by
-
Research progress on FSH-FSHR signaling in the pathogenesis of non-reproductive diseases.Front Cell Dev Biol. 2024 Nov 20;12:1506450. doi: 10.3389/fcell.2024.1506450. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39633710 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

