Fatty acid composition and biophysical characteristics of the cell membrane of feline spermatozoa
- PMID: 38702489
- PMCID: PMC11068737
- DOI: 10.1038/s41598-024-61006-5
Fatty acid composition and biophysical characteristics of the cell membrane of feline spermatozoa
Erratum in
-
Author Correction: Fatty acid composition and biophysical characteristics of the cell membrane of feline spermatozoa.Sci Rep. 2024 May 15;14(1):11109. doi: 10.1038/s41598-024-62067-2. Sci Rep. 2024. PMID: 38750209 Free PMC article. No abstract available.
Abstract
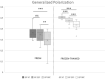
Sperm membrane composition and biophysical characteristics play a pivotal role in many physiological processes (i.e. sperm motility, capacitation, acrosome reaction and fusion with the oocyte) as well as in semen processing (e.g. cryopreservation). The aim of this study was to characterize the fatty acid content and biophysical characteristics (anisotropy, generalized polarization) of the cell membrane of domestic cat spermatozoa. Semen was collected from 34 adult male cats by urethral catheterization. After a basic semen evaluation, the fatty acid content of some of the samples (n = 11) was evaluated by gas chromatography. Samples from other individuals (n = 23) were subjected to biophysical analysis: membrane anisotropy (which is inversely proportional to membrane fluidity) and generalized polarization (describing lipid order); both measured by fluorimetry at three temperature points: 38 °C, 25 °C and 5 °C. Spermatozoa from some samples (n = 10) were cryopreserved in TRIS egg yolk-glycerol extender and underwent the same biophysical analysis after thawing. Most fatty acids in feline spermatozoa were saturated (69.76 ± 24.45%), whereas the polyunsaturated fatty acid (PUFA) content was relatively low (6.12 ± 5.80%). Lowering the temperature caused a significant decrease in membrane fluidity and an increase in generalized polarization in fresh spermatozoa, and these effects were even more pronounced following cryopreservation. Anisotropy at 38 °C in fresh samples showed strong positive correlations with viability and motility parameters after thawing. In summary, feline spermatozoa are characterized by a very low PUFA content and a low ratio of unsaturated:saturated fatty acids, which may contribute to low oxidative stress. Cryopreservation alters the structure of the sperm membrane, increasing the fluidity of the hydrophobic portion of the bilayer and the lipid order in the hydrophilic portion. Because lower membrane fluidity in fresh semen was linked with better viability and motility after cryopreservation, this parameter may be considered an important factor in determination of sperm cryoresistance.
Keywords: Domestic cat; Fatty acids; Membrane fluidity; Membrane generalized potential; Semen.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Comparative analysis of in vitro characteristics of fresh and frozen-thawed urethral and epididymal spermatozoa from cats (Felis domesticus).Theriogenology. 2016 Nov;86(8):2063-72. doi: 10.1016/j.theriogenology.2016.07.002. Epub 2016 Jul 13. Theriogenology. 2016. PMID: 27501869
-
Membrane fluidity predicts the outcome of cryopreservation of human spermatozoa.Hum Reprod. 2000 Oct;15(10):2160-4. doi: 10.1093/humrep/15.10.2160. Hum Reprod. 2000. PMID: 11006192
-
Cryopreservation of epididymal cat spermatozoa: effects of in vitro antioxidative enzymes supplementation and lipid peroxidation induction.Theriogenology. 2010 May;73(8):1076-87. doi: 10.1016/j.theriogenology.2010.01.007. Epub 2010 Mar 12. Theriogenology. 2010. PMID: 20219240
-
Recent advances in cryotolerance biomarkers for semen preservation in frozen form-A systematic review.PLoS One. 2024 May 22;19(5):e0303567. doi: 10.1371/journal.pone.0303567. eCollection 2024. PLoS One. 2024. PMID: 38776323 Free PMC article.
-
Polyunsaturated Fatty Acids in Male Ruminant Reproduction - A Review.Asian-Australas J Anim Sci. 2017 May;30(5):622-637. doi: 10.5713/ajas.15.1034. Epub 2016 Mar 4. Asian-Australas J Anim Sci. 2017. PMID: 26954196 Free PMC article. Review.
Cited by
-
Coenzyme Q10 Improves the Post-Thaw Sperm Quality in Dwarf Surfclam Mulinia lateralis.Antioxidants (Basel). 2024 Sep 4;13(9):1085. doi: 10.3390/antiox13091085. Antioxidants (Basel). 2024. PMID: 39334744 Free PMC article.
References
-
- Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochem. Biophys. Acta. 2000;1469:197–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

