Equitable implementation of a precision digital health program for glucose management in individuals with newly diagnosed type 1 diabetes
- PMID: 38702523
- PMCID: PMC11847559
- DOI: 10.1038/s41591-024-02975-y
Equitable implementation of a precision digital health program for glucose management in individuals with newly diagnosed type 1 diabetes
Abstract
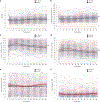
Few young people with type 1 diabetes (T1D) meet glucose targets. Continuous glucose monitoring improves glycemia, but access is not equitable. We prospectively assessed the impact of a systematic and equitable digital-health-team-based care program implementing tighter glucose targets (HbA1c < 7%), early technology use (continuous glucose monitoring starts <1 month after diagnosis) and remote patient monitoring on glycemia in young people with newly diagnosed T1D enrolled in the Teamwork, Targets, Technology, and Tight Control (4T Study 1). Primary outcome was HbA1c change from 4 to 12 months after diagnosis; the secondary outcome was achieving the HbA1c targets. The 4T Study 1 cohort (36.8% Hispanic and 35.3% publicly insured) had a mean HbA1c of 6.58%, 64% with HbA1c < 7% and mean time in the range (70-180 mg dl-1) of 68% at 1 year after diagnosis. Clinical implementation of the 4T Study 1 met the prespecified primary outcome and improved glycemia without unexpected serious adverse events. The strategies in the 4T Study 1 can be used to implement systematic and equitable care for individuals with T1D and translate to care for other chronic diseases. ClinicalTrials.gov registration: NCT04336969 .
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
D.M.M., D.S., P.P., K.H. and R.J. have received support from Stanford MCHRI, Stanford HAI and the NSF. D.S., R.J., D.M.M., K.H., D.P.Z. and A.A. have received funding from the Helmsley Charitable Trust. J.F. has received support from an NSF grant. D.P.Z. has received speakers honoraria from Medtronic Diabetes, Ascensia Diabetes, and Insulet Canada and Dexcom Canada, as well as research support from the ISPAD-Juvenile Diabetes Research Foundation Research Fellowship. D.M.M. has had research support from the NIH and his institution has received research support from Dexcom. D.M.M. has consulted for Abbott, the Helmsley Charitable Trust, Lifescan, Sanofi, Medtronic, Provention Bio, Kriya, Biospex and Bayer. K.H. has received research support from Dexcom for investigator-initiated research, and consultant fees from the Lilly Innovation Center, LifeScan Diabetes Institute and MedIQ. He has also received consulting fees from Sanofi Health and Cecelia Health. D.S. is an adviser to Carta Health. A.A. has received research support from the NIH. The other authors declare no competing interests.
Figures



References
-
- Chronic diseases in America. Centers for Disease Control and Prevention www.cdc.gov/chronicdisease/resources/infographic/chronic-diseases.htm (2022).
-
- Management of chronic conditions in schools. American Academy of Pediatrics www.aap.org/en/patient-care/school-health/management-of-chronic-conditio... (2023).
-
- Wagner EH Chronic disease management: what will it take to improve care for chronic illness? Eff. Clin. Pract. 1, 2–4 (1998). - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

