The effect of a fermented soy beverage among patients with localized prostate cancer prior to radical prostatectomy
- PMID: 38702664
- PMCID: PMC11067086
- DOI: 10.1186/s12894-024-01483-y
The effect of a fermented soy beverage among patients with localized prostate cancer prior to radical prostatectomy
Abstract
Background: Fermented soy products have shown to possess inhibitory effects on prostate cancer (PCa). We evaluated the effect of a fermented soy beverage (Q-Can®), containing medium-chain triglycerides, ketones and soy isoflavones, among men with localized PCa prior to radical prostatectomy.
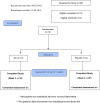
Methods: We conducted a placebo-controlled, double-blind randomized trial of Q-Can®. Stratified randomization (Cancer of the Prostate Risk Assessment (CAPRA) score at diagnosis) was used to assign patients to receive Q-Can® or placebo for 2-5 weeks before RP. Primary endpoint was change in serum PSA from baseline to end-of-study. We assessed changes in other clinical and pathologic endpoints. The primary ITT analysis compared PSA at end-of-study between randomization arms using repeated measures linear mixed model incorporating baseline CAPRA risk strata.
Results: We randomized 19 patients, 16 were eligible for analysis of the primary outcome. Mean age at enrollment was 61, 9(56.2%) were classified as low and intermediate risk, and 7(43.8%) high CAPRA risk. Among patients who received Q-Can®, mean PSA at baseline and end-of-study was 8.98(standard deviation, SD 4.07) and 8.02ng/mL(SD 3.99) compared with 8.66(SD 2.71) to 9.53ng/mL(SD 3.03), respectively, (Difference baseline - end-of-study, p = 0.36). There were no significant differences in Gleason score, clinical stage, surgical margin status, or CAPRA score between treatment arms (p > 0.05), and no significant differences between treatment arms in end-of-study or change in lipids, testosterone and FACT-P scores (p > 0.05).
Conclusions: Short exposure to Q-Can® among patients with localized PCa was not associated with changes in PSA levels, PCa characteristics including grade and stage or serum testosterone. Due to early termination from inability to recruit, study power, was not achieved.
Keywords: Fermented soy; PSA; Prostate cancer; Randomized controlled trial.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics. 2022. CA: a cancer journal for clinicians. 2022;72(1):7–33. Epub 20220112. 10.3322/caac.21708. PubMed PMID: 35020204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous