Chandipura viral glycoprotein (CNV-G) promotes Gectosome generation and enables delivery of intracellular therapeutics
- PMID: 38702887
- PMCID: PMC11287003
- DOI: 10.1016/j.ymthe.2024.04.034
Chandipura viral glycoprotein (CNV-G) promotes Gectosome generation and enables delivery of intracellular therapeutics
Abstract
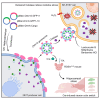
Overexpression of vesicular stomatitis virus G protein (VSV-G) elevates the secretion of EVs known as gectosomes, which contain VSV-G. Such vesicles can be engineered to deliver therapeutic macromolecules. We investigated viral glycoproteins from several viruses for their potential in gectosome production and intracellular cargo delivery. Expression of the viral glycoprotein (viral glycoprotein from the Chandipura virus [CNV-G]) from the human neurotropic pathogen Chandipura virus in 293T cells significantly augments the production of CNV-G-containing gectosomes. In comparison with VSV-G gectosomes, CNV-G gectosomes exhibit heightened selectivity toward specific cell types, including primary cells and tumor cell lines. Consistent with the differential tropism between CNV-G and VSV-G gectosomes, cellular entry of CNV-G gectosome is independent of the Low-density lipoprotein receptor, which is essential for VSV-G entry, and shows varying sensitivity to pharmacological modulators. CNV-G gectosomes efficiently deliver diverse intracellular cargos for genomic modification or responses to stimuli in vitro and in the brain of mice in vivo utilizing a split GFP and chemical-induced dimerization system. Pharmacokinetics and biodistribution analyses support CNV-G gectosomes as a versatile platform for delivering macromolecular therapeutics intracellularly.
Keywords: CNV-G; Chandipura virus; VSV-G; biodistribution; catalase; exosomes; gectosomes; genome editing; macromolecule delivery; pharmacokinetics.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The University of Colorado-Boulder currently holds the PCT patents for the use of gectosome technology, with X.Z., Q.X., Z.L., and X.L. as inventors. X.L. is a co-founder and member of the Scientific Advisory Board of OnKure Inc., which has no relationships or competing interests to this study. X.L. owns equity in OnKure Inc. X.L. is also a co-founder and interim president of Vesicle Therapeutics, Inc, a startup company that licensed gectosome technology from the University of Colorado-Boulder. X, L., X.Z., and Q.X. own equity in Vesicle Therapeutics, Inc.
Figures



References
-
- Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. - PubMed
-
- Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. - PubMed
-
- Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

