Identification of the circRNA-miRNA-mRNA network for treating methamphetamine-induced relapse and behavioral sensitization with cannabidiol
- PMID: 38702929
- PMCID: PMC11069028
- DOI: 10.1111/cns.14737
Identification of the circRNA-miRNA-mRNA network for treating methamphetamine-induced relapse and behavioral sensitization with cannabidiol
Abstract
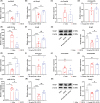
Aims: This study aims to investigate the pharmacological effects and the underlying mechanism of cannabidiol (CBD) on methamphetamine (METH)-induced relapse and behavioral sensitization in male mice.
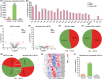
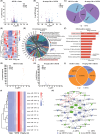
Methods: The conditioned place preference (CPP) test with a biased paradigm and open-field test were used to assess the effects of CBD on METH-induced relapse and behavioral sensitization in male mice. RNA sequencing and bioinformatics analysis was employed to identify differential expressed (DE) circRNAs, miRNAs, and mRNAs in the nucleus accumbens (NAc) of mice, and the interaction among them was predicted using competing endogenous RNAs (ceRNAs) network analysis.
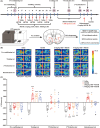
Results: Chronic administration of CBD (40 mg/kg) during the METH withdrawal phase alleviated METH (2 mg/kg)-induced CPP reinstatement and behavioral sensitization in mice, as well as mood and cognitive impairments following behavioral sensitization. Furthermore, 42 DEcircRNAs, 11 DEmiRNAs, and 40 DEmRNAs were identified in the NAc of mice. The circMeis2-miR-183-5p-Kcnj5 network in the NAc of mice is involved in the effects of CBD on METH-induced CPP reinstatement and behavioral sensitization.
Conclusions: This study constructed the ceRNAs network for the first time, revealing the potential mechanism of CBD in treating METH-induced CPP reinstatement and behavioral sensitization, thus advancing the application of CBD in METH use disorders.
Keywords: behavioral sensitization; cannabidiol; competing endogenous RNAs; methamphetamine; relapse.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Cannabidiol enhanced the development of sensitization to the expression of methamphetamine-induced conditioned place preference in male rats.J Psychiatr Res. 2021 May;137:260-265. doi: 10.1016/j.jpsychires.2021.02.045. Epub 2021 Mar 9. J Psychiatr Res. 2021. PMID: 33725638
-
The potential involvement of miR-204-3p-axon guidance network in methamphetamine-induced locomotor sensitization of mice.Neurosci Lett. 2019 Aug 10;707:134303. doi: 10.1016/j.neulet.2019.134303. Epub 2019 May 30. Neurosci Lett. 2019. PMID: 31153969
-
Regulation of miR-128 in the nucleus accumbens affects methamphetamine-induced behavioral sensitization by modulating proteins involved in neuroplasticity.Addict Biol. 2021 Jan;26(1):e12881. doi: 10.1111/adb.12881. Epub 2020 Feb 14. Addict Biol. 2021. PMID: 32058631
-
Cannabidiol Treatment Might Promote Resilience to Cocaine and Methamphetamine Use Disorders: A Review of Possible Mechanisms.Molecules. 2019 Jul 16;24(14):2583. doi: 10.3390/molecules24142583. Molecules. 2019. PMID: 31315244 Free PMC article. Review.
-
Neuropsychotoxicity of abused drugs: involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents.J Pharmacol Sci. 2008 Jan;106(1):9-14. doi: 10.1254/jphs.fm0070139. Epub 2008 Jan 16. J Pharmacol Sci. 2008. PMID: 18198472 Review.
References
-
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276‐284. - PubMed
-
- Drug situation in China. Office of China National Narcotics Control Commission. 2021. http://www.nncc626.com/2023‐06/21/c_1212236289_3.htm
-
- World Drug Report . United Nations: Office on Drugs and Crime. 2023. //www.unodc.org/unodc/en/data‐and‐analysis/world‐drug‐report‐2023.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

