Hippocampal aggregation signatures of pathogenic UBQLN2 in amyotrophic lateral sclerosis and frontotemporal dementia
- PMID: 38703371
- PMCID: PMC11449146
- DOI: 10.1093/brain/awae140
Hippocampal aggregation signatures of pathogenic UBQLN2 in amyotrophic lateral sclerosis and frontotemporal dementia
Abstract
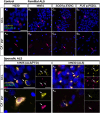
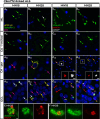
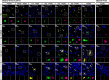
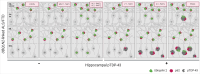
Pathogenic variants in the UBQLN2 gene cause X-linked dominant amyotrophic lateral sclerosis and/or frontotemporal dementia characterized by ubiquilin 2 aggregates in neurons of the motor cortex, hippocampus and spinal cord. However, ubiquilin 2 neuropathology is also seen in sporadic and familial amyotrophic lateral sclerosis and/or frontotemporal dementia cases not caused by UBQLN2 pathogenic variants, particularly C9orf72-linked cases. This makes the mechanistic role of mutant ubiquilin 2 protein and the value of ubiquilin 2 pathology for predicting genotype unclear. Here we examine a cohort of 44 genotypically diverse amyotrophic lateral sclerosis cases with or without frontotemporal dementia, including eight cases with UBQLN2 variants [resulting in p.S222G, p.P497H, p.P506S, p.T487I (two cases) and p.P497L (three cases)]. Using multiplexed (five-label) fluorescent immunohistochemistry, we mapped the co-localization of ubiquilin 2 with phosphorylated TDP-43, dipeptide repeat aggregates and p62 in the hippocampus of controls (n = 6), or amyotrophic lateral sclerosis with or without frontotemporal dementia in sporadic (n = 20), unknown familial (n = 3), SOD1-linked (n = 1), FUS-linked (n = 1), C9orf72-linked (n = 5) and UBQLN2-linked (n = 8) cases. We differentiate between (i) ubiquilin 2 aggregation together with phosphorylated TDP-43 or dipeptide repeat proteins; and (ii) ubiquilin 2 self-aggregation promoted by UBQLN2 pathogenic variants that cause amyotrophic lateral sclerosis and/or frontotemporal dementia. Overall, we describe a hippocampal protein aggregation signature that fully distinguishes mutant from wild-type ubiquilin 2 in amyotrophic lateral sclerosis with or without frontotemporal dementia, whereby mutant ubiquilin 2 is more prone than wild-type to aggregate independently of driving factors. This neuropathological signature can be used to assess the pathogenicity of UBQLN2 gene variants and to understand the mechanisms of UBQLN2-linked disease.
Keywords: UBQLN2; amyotrophic lateral sclerosis (ALS); frontotemporal dementia (FTD); hippocampus; neuropathology; ubiquilin 2.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

