miR-369-3p ameliorates diabetes-associated atherosclerosis by regulating macrophage succinate-GPR91 signalling
- PMID: 38703377
- PMCID: PMC11587565
- DOI: 10.1093/cvr/cvae102
miR-369-3p ameliorates diabetes-associated atherosclerosis by regulating macrophage succinate-GPR91 signalling
Abstract
Aims: Diabetes leads to dysregulated macrophage immunometabolism, contributing to accelerated atherosclerosis progression. Identifying critical factors to restore metabolic alterations and promote resolution of inflammation remains an unmet goal. MicroRNAs orchestrate multiple signalling events in macrophages, yet their therapeutic potential in diabetes-associated atherosclerosis remains unclear.
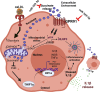
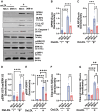
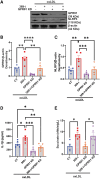
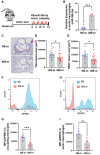
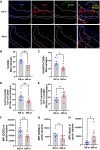
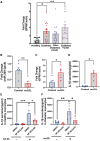
Methods and results: miRNA profiling revealed significantly lower miR-369-3p expression in aortic intimal lesions from Ldlr-/- mice on a high-fat sucrose-containing (HFSC) diet for 12 weeks. miR-369-3p was also reduced in peripheral blood mononuclear cells from diabetic patients with coronary artery disease (CAD). Cell-type expression profiling showed miR-369-3p enrichment in aortic macrophages. In vitro, oxLDL treatment reduced miR-369-3p expression in mouse bone marrow-derived macrophages (BMDMs). Metabolic profiling in BMDMs revealed that miR-369-3p overexpression blocked the oxidized low density lipoprotein (oxLDL)-mediated increase in the cellular metabolite succinate and reduced mitochondrial respiration (OXPHOS) and inflammation [Interleukin (lL)-1β, TNF-α, and IL-6]. Mechanistically, miR-369-3p targeted the succinate receptor (GPR91) and alleviated the oxLDL-induced activation of inflammasome signalling pathways. Therapeutic administration of miR-369-3p mimics in HFSC-fed Ldlr-/- mice reduced GPR91 expression in lesional macrophages and diabetes-accelerated atherosclerosis, evident by a decrease in plaque size and pro-inflammatory Ly6Chi monocytes. RNA-Seq analyses showed more pro-resolving pathways in plaque macrophages from miR-369-3p-treated mice, consistent with an increase in macrophage efferocytosis in lesions. Finally, a GPR91 antagonist attenuated oxLDL-induced inflammation in primary monocytes from human subjects with diabetes.
Conclusion: These findings establish a therapeutic role for miR-369-3p in halting diabetes-associated atherosclerosis by regulating GPR91 and macrophage succinate metabolism.
Keywords: Atherosclerosis; Diabetes; GPR91; Macrophage; Succinate; microRNA.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared.
Figures



References
-
- Haffner SM. Diabetes, hyperlipidemia, and coronary artery disease. Am J Cardiol 1999;83:17F–21F. - PubMed
-
- Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol 1999;84:11J–14J. - PubMed
-
- Armitage J, Bowman L. Cardiovascular outcomes among participants with diabetes in the recent large statin trials. Curr Opin Lipidol 2004;15:439–446. - PubMed
-
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002;23:599–622. - PubMed
-
- Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes 2004;53:3217–3225. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

