Long-Acting Injectable Cabotegravir and Rilpivirine in a Pregnant Woman With HIV
- PMID: 38703388
- PMCID: PMC11650856
- DOI: 10.1093/cid/ciae242
Long-Acting Injectable Cabotegravir and Rilpivirine in a Pregnant Woman With HIV
Abstract
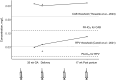
This case report describes the effects of bimonthly long-acting injectable cabotegravir (CAB)/RPV before and throughout pregnancy. CAB concentrations were comparable to those in nonpregnant individuals; RPV concentrations were 70%-75% lower. No virologic failure or vertical transmission occurred. Despite placental transfer, no congenital malformations were noted. Bimonthly long-acting injectable CAB/RPV may not be suitable for pregnant women, and monitoring of exposed infants is warranted.
Keywords: HIV; cabotegravir; long-acting injectables; pregnancy; rilpivirine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. L. v. d. W. P. received research grants from Merck and honorarium for lectures from ViiV Healthcare, all paid to the institution. D. B. received research grants and honorarium for lectures from ViiV Healthcare, all paid to the institution. A. C. received research grants from ViiV Healthcare, Gilead, Merck, all paid to the institution. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Department of Health and Human Services (DHHS) . Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States 2023. Updated 31 January 2024. Available at: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new-guidelines. Accessed November 2023.
-
- Patel P, Ford SL, Baker M, et al. Pregnancy outcomes and pharmacokinetics in pregnant women living with HIV exposed to long-acting cabotegravir and rilpivirine in clinical trials. HIV Med 2023; 24:568–79. - PubMed
-
- D'Amico R, Cenoz Gomis S, Moodley R, et al. Compassionate use of long-acting cabotegravir plus rilpivirine for people living with HIV-1 in need of parenteral antiretroviral therapy. HIV Med 2023; 24:202–11. - PubMed
-
- Committee Antiretroviral Pregnancy Registry (APR) . Antiretroviral Pregnancy Registry international interim report for 1 January 1989–31 January 2021. Available at: http://www.apregistry.com/forms/interim_report.pdf. Accessed November 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

