Tetrahydroxy stilbene glucoside rejuvenates aging hematopoietic stem cells with predilection for lymphoid differentiation via AMPK and Tet2
- PMID: 38704089
- PMCID: PMC11976424
- DOI: 10.1016/j.jare.2024.04.027
Tetrahydroxy stilbene glucoside rejuvenates aging hematopoietic stem cells with predilection for lymphoid differentiation via AMPK and Tet2
Abstract
Introduction: Aging of hematopoietic stem cells (HSCs) has emerged as an important challenge to human health. Recent advances have raised the prospect of rejuvenating aging HSCs via specific medical interventions, including pharmacological treatments. Nonetheless, efforts to develop such drugs are still in infancy until now.
Objectives: We aimed to screen the prospective agents that can rejuvenate aging HSCs and explore the potential mechanisms.
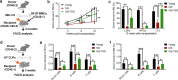
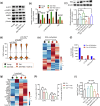
Methods: We screened a set of natural anti-aging compounds through oral administration to sub-lethally irradiated mice, and identified 2,3,5,4'-tetrahydroxystilbene-2-O-β-D-glucoside (TSG) as a potent rejuvenating agent for aging HSCs. Then naturally aged mice were used for the follow-up assessment to determine the HSC rejuvenating potential of TSG. Finally, based on the transcriptome and DNA methylation analysis, we validated the role of the AMP-activated protein kinase (AMPK)-ten-eleven-translocation 2 (Tet2) axis (the AMPK-Tet2 axis) as the underlying mechanisms of TSG for ameliorating HSCs aging.
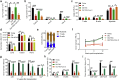
Results: TSG treatment not only significantly increased the absolute number of common lymphoid progenitors (CLPs) along with B lymphocytes, but also boosted the HSCs/CLPs repopulation potential of aging mice. Further elaborated mechanism research demonstrated that TSG supplementation restored the stemness of aging HSCs, as well as promoted an epigenetic reprograming that was associated with an improved regenerative capacity and an increased rate of lymphopoiesis. Such effects were diminished when the mice were co-treated with an AMPK inhibitor, or when it was performed in Tet2 knockout mice as well as senescent cells assay.
Conclusion: TSG is effective in rejuvenating aging HSCs by modulating the AMPK- Tet2 axis and thus represents a potential candidate for developing effective HSC rejuvenating therapies.
Keywords: AMPK-Tet2 axis; Aging; Hematopoietic stem cells; Lymphoid differentiation; Tetrahydroxy stilbene glucoside.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Pang W.W., Schrier S.L., Weissman I.L. Age-associated changes in human hematopoietic stem cells. Semin Hematol. 2017;54:39–42. - PubMed
-
- Geiger H., de Haan G., Florian M.C. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13:376–389. - PubMed
-
- de Haan G., Lazare S.S. Aging of hematopoietic stem cells. Blood. 2018;131:479–487. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

