IL-4Rα signaling promotes barrier-altering oncostatin M and IL-6 production in aspirin-exacerbated respiratory disease
- PMID: 38704098
- PMCID: PMC11305950
- DOI: 10.1016/j.jaci.2024.04.020
IL-4Rα signaling promotes barrier-altering oncostatin M and IL-6 production in aspirin-exacerbated respiratory disease
Abstract
Background: Aspirin-exacerbated respiratory disease (AERD) is a severe disease involving dysregulated type 2 inflammation. However, the role other inflammatory pathways play in AERD is poorly understood.
Objective: We sought to broadly define the inflammatory milieu of the upper respiratory tract in AERD and to determine the effects of IL-4Rα inhibition on mediators of nasal inflammation.
Methods: Twenty-two AERD patients treated with dupilumab for 3 months were followed over 3 visits and compared to 10 healthy controls. Nasal fluid was assessed for 45 cytokines and chemokines using Olink Target 48. Blood neutrophils and cultured human mast cells, monocytes/macrophages, and nasal fibroblasts were assessed for response to IL-4/13 stimulation in vitro.
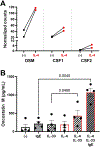
Results: Of the nasal fluid cytokines measured, nearly one third were higher in AERD patients compared to healthy controls, including IL-6 and the IL-6 family-related cytokine oncostatin M (OSM), both of which correlated with nasal albumin levels, a marker of epithelial barrier dysregulation. Dupilumab significantly decreased many nasal mediators, including OSM and IL-6. IL-4 stimulation induced OSM production from mast cells and macrophages but not from neutrophils, and OSM and IL-13 stimulation induced IL-6 production from nasal fibroblasts.
Conclusion: In addition to type 2 inflammation, innate and IL-6-related cytokines are also elevated in the respiratory tract in AERD. Both OSM and IL-6 are locally produced in nasal polyps and likely promote pathology by negatively affecting epithelial barrier function. IL-4Rα blockade, although seemingly directed at type 2 inflammation, also decreases mediators of innate inflammation and epithelial dysregulation, which may contribute to dupilumab's therapeutic efficacy in AERD.
Keywords: AERD; Aspirin-exacerbated respiratory disease; dupilumab; interleukin 13; interleukin 4; interleukin 4Rα; interleukin 6; mast cells; nasal polyp; oncostatin M.
Copyright © 2024 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement Supported by the National Institutes of Health (grants U19AI095219 and K23AI139352), by the Immune Tolerance Network (AI109565-07), and by generous contributions from the Vinik and Kaye families. Disclosure of potential conflict of interest: T. Laidlaw has served on scientific advisory boards for GlaxoSmithKline, AstraZeneca, Sanofi-Genzyme, Regeneron, and Eli Lilly. K. Buchheit has served on scientific advisory boards for AstraZeneca, Sanofi-Genzyme, Regeneron, and GlaxoSmithKline; and has received personal consulting fees from Genentech. D. Dwyer receives research funding from Blueprint Medicines and has received personal consulting fees from Celldex Therapeutics. J. Boyce serves on the scientific advisory boards of Siolta Therapeutics, Third Harmonic Bio, and Jasper Therapeutics. The rest of the authors declare that they have no relevant conflicts of interest.
Figures
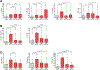
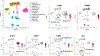



Similar articles
-
Reduced ALDH2 in the respiratory tract associates with dysregulated alcohol metabolism and respiratory reactions in AERD.J Allergy Clin Immunol. 2025 Aug 28:S0091-6749(25)00893-0. doi: 10.1016/j.jaci.2025.08.011. Online ahead of print. J Allergy Clin Immunol. 2025. PMID: 40885289
-
Oral and intranasal aspirin desensitisation for non-steroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease.Cochrane Database Syst Rev. 2025 Jan 7;1(1):CD013476. doi: 10.1002/14651858.CD013476.pub2. Cochrane Database Syst Rev. 2025. PMID: 39775459
-
[Guidelines for the prevention and management of bronchial asthma (2024 edition)].Zhonghua Jie He He Hu Xi Za Zhi. 2025 Mar 12;48(3):208-248. doi: 10.3760/cma.j.cn112147-20241013-00601. Zhonghua Jie He He Hu Xi Za Zhi. 2025. PMID: 40050074 Chinese.
-
Oncostatin M Drives Th2 Polarized Allergic Airway Inflammation Through Fibroblast Reprogramming and Endoplasmic Reticulum Stress.Int J Nanomedicine. 2025 Jul 14;20:9019-9030. doi: 10.2147/IJN.S535265. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40689016 Free PMC article.
-
Systemic treatments for eczema: a network meta-analysis.Cochrane Database Syst Rev. 2020 Sep 14;9(9):CD013206. doi: 10.1002/14651858.CD013206.pub2. Cochrane Database Syst Rev. 2020. PMID: 32927498 Free PMC article.
Cited by
-
Global research trends and hotspots in aspirin studies (2014-2024): a bibliometric perspective.Front Pharmacol. 2025 May 16;16:1513318. doi: 10.3389/fphar.2025.1513318. eCollection 2025. Front Pharmacol. 2025. PMID: 40453660 Free PMC article. Review.
-
Clinical efficacy and mechanisms of biologics for chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2025 May;155(5):1401-1410. doi: 10.1016/j.jaci.2025.03.011. Epub 2025 Mar 23. J Allergy Clin Immunol. 2025. PMID: 40132672 Review.
-
Clinical and mechanistic advancements in aspirin exacerbated respiratory disease.J Allergy Clin Immunol. 2025 May;155(5):1411-1419. doi: 10.1016/j.jaci.2025.03.006. Epub 2025 Mar 18. J Allergy Clin Immunol. 2025. PMID: 40113018 Review.
-
New insights into the mechanisms of aspirin-exacerbated respiratory disease.Curr Opin Allergy Clin Immunol. 2025 Feb 1;25(1):41-46. doi: 10.1097/ACI.0000000000001051. Epub 2024 Dec 6. Curr Opin Allergy Clin Immunol. 2025. PMID: 39641750 Review.
-
The Direct and Indirect Role of IgE on Airway Epithelium in Asthma.Allergy. 2025 Apr;80(4):919-931. doi: 10.1111/all.16459. Epub 2025 Feb 18. Allergy. 2025. PMID: 39963805 Free PMC article. Review.
References
-
- Samter M, Beers RF Jr. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68(5):975–83. - PubMed
-
- Kim JE, Kountakis SE. The prevalence of Samter’s triad in patients undergoing functional endoscopic sinus surgery. Ear Nose Throat J. 2007;86(7):396–9. - PubMed
-
- Jakiela B, Soja J, Sladek K, Przybyszowski M, Plutecka H, Gielicz A, et al. Heterogeneity of lower airway inflammation in patients with NSAID-exacerbated respiratory disease. J Allergy Clin Immunol. 2021;147(4):1269–80. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

