Targeting senescent cells with NKG2D-CAR T cells
- PMID: 38704364
- PMCID: PMC11069534
- DOI: 10.1038/s41420-024-01976-7
Targeting senescent cells with NKG2D-CAR T cells
Abstract
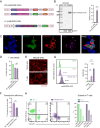
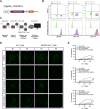
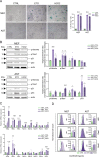
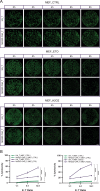
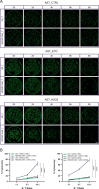
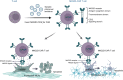
This study investigates the efficacy of NKG2D chimeric antigen receptor (CAR) engineered T cells in targeting and eliminating stress-induced senescent cells in vitro. Cellular senescence contributes to age-related tissue decline and is characterized by permanent cell cycle arrest and the senescence-associated secretory phenotype (SASP). Immunotherapy, particularly CAR-T cell therapy, emerges as a promising approach to selectively eliminate senescent cells. Our focus is on the NKG2D receptor, which binds to ligands (NKG2DLs) upregulated in senescent cells, offering a target for CAR-T cells. Using mouse embryonic fibroblasts (MEFs) and astrocytes (AST) as senescence models, we demonstrate the elevated expression of NKG2DLs in response to genotoxic and oxidative stress. NKG2D-CAR T cells displayed potent cytotoxicity against these senescent cells, with minimal effects on non-senescent cells, suggesting their potential as targeted senolytics. In conclusion, our research presents the first evidence of NKG2D-CAR T cells' ability to target senescent brain cells, offering a novel approach to manage senescence-associated diseases. The findings pave the way for future investigations into the therapeutic applicability of NKG2D-targeting CAR-T cells in naturally aged organisms and models of aging-associated brain diseases in vivo.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

