The emerging role of regulated cell death in ischemia and reperfusion-induced acute kidney injury: current evidence and future perspectives
- PMID: 38704372
- PMCID: PMC11069531
- DOI: 10.1038/s41420-024-01979-4
The emerging role of regulated cell death in ischemia and reperfusion-induced acute kidney injury: current evidence and future perspectives
Abstract
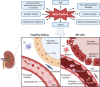
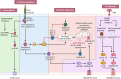
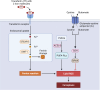
Renal ischemia‒reperfusion injury (IRI) is one of the main causes of acute kidney injury (AKI), which is a potentially life-threatening condition with a high mortality rate. IRI is a complex process involving multiple underlying mechanisms and pathways of cell injury and dysfunction. Additionally, various types of cell death have been linked to IRI, including necroptosis, apoptosis, pyroptosis, and ferroptosis. These processes operate differently and to varying degrees in different patients, but each plays a role in the various pathological conditions of AKI. Advances in understanding the underlying pathophysiology will lead to the development of new therapeutic approaches that hold promise for improving outcomes for patients with AKI. This review provides an overview of the recent research on the molecular mechanisms and pathways underlying IRI-AKI, with a focus on regulated cell death (RCD) forms such as necroptosis, pyroptosis, and ferroptosis. Overall, targeting RCD shows promise as a potential approach to treating IRI-AKI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
[The role of regulated cell death in renal ischemia-reperfusion injury].Sheng Li Xue Bao. 2022 Apr 25;74(2):320-332. Sheng Li Xue Bao. 2022. PMID: 35503080 Review. Chinese.
-
[Acute kidney injury and regulated cell death].Sheng Li Xue Bao. 2022 Feb 25;74(1):4-14. Sheng Li Xue Bao. 2022. PMID: 35199121 Chinese.
-
Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research.J Hematol Oncol. 2022 Dec 8;15(1):174. doi: 10.1186/s13045-022-01392-3. J Hematol Oncol. 2022. PMID: 36482419 Free PMC article. Review.
-
Potential effects of different cell death inhibitors in protecting against ischemia-reperfusion injury in steatotic liver.Int Immunopharmacol. 2024 Feb 15;128:111545. doi: 10.1016/j.intimp.2024.111545. Epub 2024 Jan 19. Int Immunopharmacol. 2024. PMID: 38244517
-
Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis.Stem Cell Res Ther. 2022 Jul 15;13(1):297. doi: 10.1186/s13287-022-02986-x. Stem Cell Res Ther. 2022. PMID: 35841017 Free PMC article.
Cited by
-
Molecular Mechanisms and Potential Therapeutic Targets of Ischemia-Reperfusion Injury in Kidney Transplantation.Curr Issues Mol Biol. 2025 Apr 17;47(4):282. doi: 10.3390/cimb47040282. Curr Issues Mol Biol. 2025. PMID: 40699682 Free PMC article. Review.
-
Multi-omics Analysis Reveals the Propagation Mechanism of Ferroptosis in Acute Kidney Injury.Inflammation. 2025 May 13. doi: 10.1007/s10753-025-02311-7. Online ahead of print. Inflammation. 2025. PMID: 40358793
-
Insights into the effects of apelin-13 on renal function and NHE3 activity following ischemia/reperfusion-induced acute kidney injury.Front Physiol. 2025 Mar 19;16:1544274. doi: 10.3389/fphys.2025.1544274. eCollection 2025. Front Physiol. 2025. PMID: 40177358 Free PMC article.
-
Long Non-coding RNA MIR22HG Alleviates Ischemic Acute Kidney Injury by Targeting the miR-134-5p/NFAT5 axis.Inflammation. 2025 Mar 17. doi: 10.1007/s10753-025-02286-5. Online ahead of print. Inflammation. 2025. PMID: 40095256
-
[Elevated neutrophil-lymphocyte ratio and delayed graft function].Rev Med Inst Mex Seguro Soc. 2025 Mar 3;63(2):e6440. doi: 10.5281/zenodo.14617087. Rev Med Inst Mex Seguro Soc. 2025. PMID: 40279262 Free PMC article. Spanish.
References
-
- KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

