Mutation characteristics and molecular evolution of ovarian metastasis from gastric cancer and potential biomarkers for paclitaxel treatment
- PMID: 38704377
- PMCID: PMC11069556
- DOI: 10.1038/s41467-024-48144-0
Mutation characteristics and molecular evolution of ovarian metastasis from gastric cancer and potential biomarkers for paclitaxel treatment
Abstract
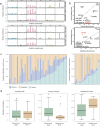
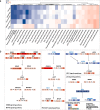
Ovarian metastasis is one of the major causes of treatment failure in patients with gastric cancer (GC). However, the genomic characteristics of ovarian metastasis in GC remain poorly understood. In this study, we enroll 74 GC patients with ovarian metastasis, with 64 having matched primary and metastatic samples. Here, we show a characterization of the mutation landscape of this disease, alongside an investigation into the molecular heterogeneity and pathway mutation enrichments between synchronous and metachronous metastasis. We classify patients into distinct clonal evolution patterns based on the distribution of mutations in paired samples. Notably, the parallel evolution group exhibits the most favorable prognosis. Additionally, by analyzing the differential response to chemotherapy, we identify potential biomarkers, including SALL4, CCDC105, and CLDN18, for predicting the efficacy of paclitaxel treatment. Furthermore, we validate that CLDN18 fusion mutations improve tumor response to paclitaxel treatment in GC with ovarian metastasis in vitro and vivo.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Jianpi Yangzheng decoction suppresses gastric cancer progression via modulating the miR-448/CLDN18.2 mediated YAP/TAZ signaling.J Ethnopharmacol. 2023 Jul 15;311:116450. doi: 10.1016/j.jep.2023.116450. Epub 2023 Apr 5. J Ethnopharmacol. 2023. PMID: 37023839
-
Phenotypic modifications in ovarian cancer stem cells following Paclitaxel treatment.Cancer Med. 2013 Dec;2(6):751-62. doi: 10.1002/cam4.115. Epub 2013 Aug 27. Cancer Med. 2013. PMID: 24403249 Free PMC article.
-
The relationships between the chemosensitivity of human gastric cancer to paclitaxel and the expressions of class III β-tubulin, MAPT, and survivin.Med Oncol. 2014 May;31(5):950. doi: 10.1007/s12032-014-0950-3. Epub 2014 Apr 11. Med Oncol. 2014. PMID: 24722794
-
Paclitaxel. An update of its use in the treatment of metastatic breast cancer and ovarian and other gynaecological cancers.Drugs Aging. 1998 Apr;12(4):305-34. doi: 10.2165/00002512-199812040-00005. Drugs Aging. 1998. PMID: 9571394 Review.
-
Landscape of systemic therapy for ovarian cancer in 2019: Primary therapy.Cancer. 2019 Dec 15;125 Suppl 24:4582-4586. doi: 10.1002/cncr.32475. Cancer. 2019. PMID: 31967679 Review.
Cited by
-
InterPAD is a database of drug-drug interaction between phytochemicals and anticancer drugs.Sci Rep. 2025 Jul 9;15(1):24616. doi: 10.1038/s41598-025-10240-6. Sci Rep. 2025. PMID: 40634546 Free PMC article.
-
Comprehensive analysis of molecular characteristics between primary and breast-derived metastatic ovarian cancer.Transl Cancer Res. 2025 Mar 30;14(3):1675-1690. doi: 10.21037/tcr-24-1441. Epub 2025 Mar 24. Transl Cancer Res. 2025. PMID: 40225000 Free PMC article.
-
Effect of Electric Fields on the Mechanical Mechanism of Regorafenib-VEGFR2 Interaction to Enhance Inhibition of Hepatocellular Carcinoma.Biomolecules. 2025 Jan 1;15(1):42. doi: 10.3390/biom15010042. Biomolecules. 2025. PMID: 39858437 Free PMC article.
-
CaMutQC: An R package for integrative quality control and filtration of cancer somatic mutations.Comput Struct Biotechnol J. 2025 Jul 16;27:3147-3154. doi: 10.1016/j.csbj.2025.07.011. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40727424 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

