Record-High Thermoelectric Performance in Al-Doped ZnO via Anderson Localization of Band Edge States
- PMID: 38704699
- PMCID: PMC11234415
- DOI: 10.1002/advs.202309291
Record-High Thermoelectric Performance in Al-Doped ZnO via Anderson Localization of Band Edge States
Abstract
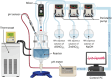
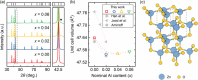
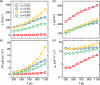
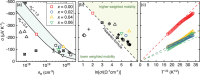
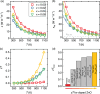
Oxides are of interest for thermoelectrics due to their high thermal stability, chemical inertness, low cost, and eco-friendly constituting elements. Here, adopting a unique synthesis route via chemical co-precipitation at strongly alkaline conditions, one of the highest thermoelectric performances for ZnO ceramics ( 21.5 µW cm-1 K-2 and 0.5 at 1100 K in ) is achieved. These results are linked to a distinct modification of the electronic structure: charge carriers become trapped at the edge of the conduction band due to Anderson localization, evidenced by an anomalously low carrier mobility, and characteristic temperature and doping dependencies of charge transport. The bi-dimensional optimization of doping and carrier localization enable a simultaneous improvement of the Seebeck coefficient and electrical conductivity, opening a novel pathway to advance ZnO thermoelectrics.
Keywords: Anderson localization; ZnO; chemical co‐precipitation; oxides; thermoelectric materials; wet chemistry.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ioffe A., Semiconductor thermoelements, and Thermoelectric cooling, Infosearch, London: 1957.
-
- Wang H., Gibbs Z. M., Takagiwa Y., Snyder G. J., Energy Environ. Sci. 2014, 7, 804.
-
- Zhao L., Wang X., Fei F. Y., Wang J., Cheng Z., Dou S., Wang J., Snyder G. J., J. Mater. Chem. A 2015, 3, 9432.
-
- Poudel B., Hao Q., Ma Y., Lan Y., Minnich A., Yu B., Yan X., Wang D., Muto A., Vashaee D., Chen X., Liu J., Dresselhaus M. S., Chen G., Ren Z., Science 2008, 320, 634. - PubMed
-
- Poudeu P. F. P., D'Angelo J., Downey A. D., Short J. L., Hogan T. P., Kanatzidis M. G., Angewandte Chemie 2006, 45, 3835. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
