Real-World Impact of Upfront Cytoreductive Nephrectomy in Metastatic Non-Clear Cell Renal Cell Carcinoma Treated with First-Line Immunotherapy Combinations or Tyrosine Kinase Inhibitors (A Sub-Analysis from the ARON-1 Retrospective Study)
- PMID: 38704759
- PMCID: PMC11230988
- DOI: 10.1007/s11523-024-01065-w
Real-World Impact of Upfront Cytoreductive Nephrectomy in Metastatic Non-Clear Cell Renal Cell Carcinoma Treated with First-Line Immunotherapy Combinations or Tyrosine Kinase Inhibitors (A Sub-Analysis from the ARON-1 Retrospective Study)
Abstract
Background: About 20% of patients with renal cell carcinoma present with non-clear cell histology (nccRCC), encompassing various histological types. While surgery remains pivotal for localized-stage nccRCC, the role of cytoreductive nephrectomy (CN) in metastatic nccRCC is contentious. Limited data exist on the role of CN in metastatic nccRCC under current standard of care.
Objective: This retrospective study focused on the impact of upfront CN on metastatic nccRCC outcomes with first-line immune checkpoint inhibitor (IO) combinations or tyrosine kinase inhibitor (TKI) monotherapy.
Methods: The study included 221 patients with nccRCC and synchronous metastatic disease, treated with IO combinations or TKI monotherapy in the first line. Baseline clinical characteristics, systemic therapy, and treatment outcomes were analyzed. The primary objective was to assess clinical outcomes, including progression-free survival (PFS) and overall survival (OS). Statistical analysis involved the Fisher exact test, Pearson's correlation coefficient, analysis of variance, Kaplan-Meier method, log-rank test, and univariate/multivariate Cox proportional hazard regression models.
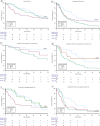
Results: Median OS for patients undergoing upfront CN was 36.8 (95% confidence interval [CI] 24.9-71.3) versus 20.8 (95% CI 12.6-24.8) months for those without CN (p = 0.005). Upfront CN was significantly associated with OS in the multivariate Cox regression analysis (hazard ratio 0.47 [95% CI 0.31-0.72], p < 0.001). In patients without CN, the median OS and PFS was 24.5 (95% CI 18.1-40.5) and 13.0 months (95% CI 6.6-23.5) for patients treated with IO+TKI versus 7.5 (95% CI 4.3-22.4) and 4.9 months (95% CI 3.0-8.1) for those receiving the IO+IO combination (p = 0.059 and p = 0.032, respectively).
Conclusions: Our study demonstrates the survival benefits of upfront CN compared with systemic therapy without CN. The study suggests that the use of IO+TKI combination or, eventually, TKI monotherapy might be a better choice than IO+IO combination for patients who are not candidates for CN regardless of IO eligibility. Prospective trials are needed to validate these findings and refine the role of CN in current mRCC management.
© 2024. The Author(s).
Conflict of interest statement
Ondrej Fiala received honoraria from Novartis, Janssen, Merck, BMS, MSD, Pierre Fabre, and Pfizer for consultations and lectures unrelated to this project. Sebastiano Buti has received honoraria for speaking at scientific events and advisory roles from AstraZeneca, BMS, Ipsen, Merck, Eisai, MSD, Novartis, and Pfizer and research funding from Novartis and Pfizer unrelated to this project. Francesco Massari has received research support and/or honoraria from Astellas, BMS, Janssen, Ipsen, MSD, and Pfizer outside the submitted work. Enrique Grande has received honoraria for speaker engagements, advisory roles, or funding of continuous medical education from Adacap, Amgen, Angelini, Astellas, AstraZeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis-Oncology, Eisai, Eusa Pharma, Genetracer, Guardant Health, HRA-Pharma, Ipsen, ITM-Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, ONCODNA (Biosequence), Palex, Pharmamar, Pierre Fabre, Pfizer, Roche, Sanofi-Genzyme, Servier, Taiho, and Thermo Fisher Scientific and research grants from Pfizer, AstraZeneca, Astellas, and Lexicon Pharmaceuticals. All of the above are unrelated to the present paper. Ugo De Giorgi services as an advisory/board member of Astellas, Bayer, BMS, Ipsen, Janssen, Merck, Pfizer, and Sanofi, has received research grant/funding to the institution from AstraZeneca, Roche, and Sanofi, and travel/accommodations/expenses from AstraZeneca, BMS, Ipsen, Janssen, and Pfizer. All of the above are unrelated to the present paper. Javier Molina-Cerrillo declares consultant, advisory, or speaker roles for Ipsen, Roche, Pfizer, Sanofi, Janssen, and BMS unrelated to this project. Zin W. Myint has received research support from Merck unrelated to the present paper. Ray Manneh Kopp has received research support and/or honoraria from Amgen, Astellas, AstraZeneca, Bayer, BMS, Eli Lilly, Janssen, MSD, Pfizer, Tecnofarma, and Roche unrelated to this project. Jakub Kucharz has received honoraria from Angelini, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, IPSEN, Janssen, Merck MSD, Novartis, and Pfizer, and research funding from Novartis, all unrelated to the present paper. Maria Giuseppa Vitale has received honoraria from Astellas, BMS, MSD, and Ipsen unrelated to this project. Alvaro Pinto has received research support and/or honoraria from Pfizer, Novartis, Ipsen, BMS, Janssen, Astellas, Sanofi, Bayer, Clovis, Roche, MSD, Pierre Fabre, Merck, Bayer, Pharmacyclics, AstraZeneca, Eisai, and Aveo unrelated to this project. Thomas Büttner has received fees for speakers bureau, travel, and accommodation from Astellas, Ipsen, and MSD, all unrelated to the present paper. Carlo Messina has received fees for speakers bureau and advisory board activities from Merck, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Astellas, AstraZeneca, Baier, and GSK, all unrelated to the present paper. Fernando Sabino M. Monteiro has received research support from Janssen and Merck Sharp Dome and honoraria from Janssen, Ipsen, Bristol Myers Squibb, and Merck Sharp Dome. All unrelated to the present paper. Ravindran Kanesvaran has received fees for speakers bureau and advisory board activities from Pfizer, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Merck, Amgen, Astellas, and Bayer, all unrelated to this project. Tomáš Büchler has received research support from AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Merck KGaA, MSD, and Novartis, consulting fees from Bristol Myers Squibb, Astellas, Janssen, and Sanofi/Aventis, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Ipsen, Bristol Myers Squibb, AstraZeneca, Roche, Servier, Accord, MSD, and Pfizer. All of the above are unrelated to the present paper. Jindřich Kopecký has received consulting fees from Bristol Myers Squibb, Novartis, Pfizer, Merck, MSD, and Ipsen and payment or honoraria for lectures, presentations, speakers bureau, manuscript writing, or educational events from Ipsen, Bristol Myers Squibb, MSD, Merck, Novartis, and Pfizer. All of the above are unrelated to the present paper. Camillo Porta has received honoraria from Angelini Pharma, AstraZeneca, BMS, Eisai, Ipsen, and MSD and acted as a Protocol Steering Committee Member for BMS, Eisai, and MSD unrelated to this project. Matteo Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas, A.A.A., and Bayer unrelated to the present paper. Aristotelis Bamias, Renate Pichler, Marco Maruzzo, Emmanuel Seront, Fabio Calabrò, Gaetano Facchini, Rossana Berardi, Luigi Formisano, Nicola Battelli, Daniele Santini, and Giulia Claire Giudice have no conflicts of interest that are directly relevant to the content of this article.
Figures


Similar articles
-
A Nationwide Real-world Evaluation of Upfront Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Renal Cell Carcinoma in the Immunotherapy Era.Eur Urol Oncol. 2025 Jun;8(3):623-631. doi: 10.1016/j.euo.2025.02.011. Epub 2025 Apr 12. Eur Urol Oncol. 2025. PMID: 40221280
-
Papillary Renal Cell Carcinoma: Outcomes for Patients Receiving First-line Immune-based Combinations or Tyrosine Kinase Inhibitors from the ARON-1 Study.Eur Urol Oncol. 2024 Oct;7(5):1123-1131. doi: 10.1016/j.euo.2024.03.011. Epub 2024 Apr 4. Eur Urol Oncol. 2024. PMID: 38575409
-
Pathological Response and Outcomes in Patients With Metastatic Renal Cell Carcinoma (mRCC) Receiving Immunotherapy-Based Therapies and Undergoing Deferred Cytoreductive Nephrectomy (CN).Clin Genitourin Cancer. 2024 Oct;22(5):102177. doi: 10.1016/j.clgc.2024.102177. Epub 2024 Jul 23. Clin Genitourin Cancer. 2024. PMID: 39218752
-
Association between cytoreductive nephrectomy and survival among patients with metastatic renal cell carcinoma receiving modern therapies: a systematic review and meta-analysis examining effect modification according to systemic therapy approach.Cancer Causes Control. 2021 Jul;32(7):675-680. doi: 10.1007/s10552-021-01435-z. Epub 2021 May 8. Cancer Causes Control. 2021. PMID: 33963938
-
Upfront Versus Deferred Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-analysis.Eur Urol Focus. 2025 Jan;11(1):100-108. doi: 10.1016/j.euf.2024.08.002. Epub 2024 Sep 16. Eur Urol Focus. 2025. PMID: 39289076
Cited by
-
Therapeutic efficacy of immune-oncology combination therapy in advanced renal cell carcinoma without prior nephrectomy.Int J Clin Oncol. 2025 Apr;30(4):770-779. doi: 10.1007/s10147-025-02710-8. Epub 2025 Feb 3. Int J Clin Oncol. 2025. PMID: 39899167
-
A novel nomogram for survival prediction in renal cell carcinoma patients with brain metastases: an analysis of the SEER database.Front Immunol. 2025 Jun 30;16:1572580. doi: 10.3389/fimmu.2025.1572580. eCollection 2025. Front Immunol. 2025. PMID: 40661946 Free PMC article.
-
mRNA expression, tumor heterogeneity, and response to therapy in patients with advanced renal cell carcinoma treated with immune-based combinations (ARON-1α).Biochem Biophys Rep. 2025 Aug 7;43:102162. doi: 10.1016/j.bbrep.2025.102162. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40821906 Free PMC article.
-
Impact of Preoperative Systemic Therapy on Cytoreductive Nephrectomy Outcomes in the National Surgical Quality Improvement Program (NSQIP).Clin Genitourin Cancer. 2025 Feb;23(1):102258. doi: 10.1016/j.clgc.2024.102258. Epub 2024 Nov 1. Clin Genitourin Cancer. 2025. PMID: 39615117
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed 24 Apr 2024.
-
- Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R, European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/S0140-6736(01)06103-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

