Quantitative profiling of cochlear synaptosomal proteins in cisplatin-induced synaptic dysfunction
- PMID: 38705005
- PMCID: PMC11116033
- DOI: 10.1016/j.heares.2024.109022
Quantitative profiling of cochlear synaptosomal proteins in cisplatin-induced synaptic dysfunction
Abstract
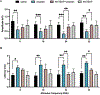
The disruption of ribbon synapses in the cochlea impairs the transmission of auditory signals from the cochlear sensory receptor cells to the auditory cortex. Although cisplatin-induced loss of ribbon synapses is well-documented, and studies have reported nitration of cochlear proteins after cisplatin treatment, yet the underlying mechanism of cochlear synaptopathy is not fully understood. This study tests the hypothesis that cisplatin treatment alters the abundance of cochlear synaptosomal proteins, and selective targeting of nitrative stress prevents the associated synaptic dysfunction. Auditory brainstem responses of mice treated with cisplatin showed a reduction in amplitude and an increase in latency of wave I, indicating cisplatin-induced synaptic dysfunction. The mass spectrometry analysis of cochlear synaptosomal proteins identified 102 proteins that decreased in abundance and 249 that increased in abundance after cisplatin treatment. Pathway analysis suggested that the dysregulated proteins were involved in calcium binding, calcium ion regulation, synapses, and endocytosis pathways. Inhibition of nitrative stress by co-treatment with MnTBAP, a peroxynitrite scavenger, attenuated cisplatin-induced changes in the abundance of 27 proteins. Furthermore, MnTBAP co-treatment prevented the cisplatin-induced decrease in the amplitude and increase in the latency of wave I. Together, these findings suggest a potential role of oxidative/nitrative stress in cisplatin-induced cochlear synaptic dysfunction.
Keywords: Cisplatin; Cochlear synaptosome; MnTBAP chloride; Nitrative stress; Ototoxicity; Synaptic dysfunction.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures




Similar articles
-
Targeting nitrative stress for attenuating cisplatin-induced downregulation of cochlear LIM domain only 4 and ototoxicity.Redox Biol. 2016 Dec;10:257-265. doi: 10.1016/j.redox.2016.10.016. Epub 2016 Oct 31. Redox Biol. 2016. PMID: 27821327 Free PMC article.
-
Assessment of cochlear toxicity in response to chronic 3,3'-iminodipropionitrile in mice reveals early and reversible functional loss that precedes overt histopathology.Arch Toxicol. 2021 Mar;95(3):1003-1021. doi: 10.1007/s00204-020-02962-5. Epub 2021 Jan 25. Arch Toxicol. 2021. PMID: 33495873 Free PMC article.
-
Lmo4 Deficiency Enhances Susceptibility to Cisplatin-Induced Cochlear Apoptosis and Hearing Loss.Mol Neurobiol. 2021 May;58(5):2019-2029. doi: 10.1007/s12035-020-02226-4. Epub 2021 Jan 7. Mol Neurobiol. 2021. PMID: 33411315 Free PMC article.
-
Audibility, speech perception and processing of temporal cues in ribbon synaptic disorders due to OTOF mutations.Hear Res. 2015 Dec;330(Pt B):200-12. doi: 10.1016/j.heares.2015.07.007. Epub 2015 Jul 15. Hear Res. 2015. PMID: 26188103 Review.
-
Transient Receptor Potential Channels and Auditory Functions.Antioxid Redox Signal. 2022 Jun;36(16-18):1158-1170. doi: 10.1089/ars.2021.0191. Epub 2021 Dec 31. Antioxid Redox Signal. 2022. PMID: 34465184 Free PMC article. Review.
Cited by
-
MnSOD Mimetics in Therapy: Exploring Their Role in Combating Oxidative Stress-Related Diseases.Antioxidants (Basel). 2024 Nov 23;13(12):1444. doi: 10.3390/antiox13121444. Antioxidants (Basel). 2024. PMID: 39765773 Free PMC article. Review.
-
MnTBAP, a peroxynitrite scavenger, attenuates cisplatin-induced apoptosis and cytotoxicity in organ of Corti cells.Toxicol Rep. 2025 Feb 18;14:101967. doi: 10.1016/j.toxrep.2025.101967. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 40046635 Free PMC article.
-
Inhibition of inner ear macrophage phagocytosis alleviates cisplatin-induced ototoxicity.Commun Biol. 2025 Jul 30;8(1):1134. doi: 10.1038/s42003-025-08525-7. Commun Biol. 2025. PMID: 40739377 Free PMC article.
-
Unraveling the molecular landscape of lead-induced cochlear synaptopathy: a quantitative proteomics analysis.Front Cell Neurosci. 2024 Jul 22;18:1408208. doi: 10.3389/fncel.2024.1408208. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39104440 Free PMC article.
-
Cisplatin-Induced Hearing Loss, Oxidative Stress, and Antioxidants as a Therapeutic Strategy-A State-of-the-Art Review.Antioxidants (Basel). 2024 Dec 21;13(12):1578. doi: 10.3390/antiox13121578. Antioxidants (Basel). 2024. PMID: 39765905 Free PMC article. Review.
References
-
- Borse V, Al Aameri RFH, Sheehan K, Sheth S, Kaur T, Mukherjea D, Tupal S, Lowy M, Ghosh S, Dhukhwa A, Bhatta P, Rybak LP, & Ramkumar V (2017). Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis, 8(7), e2921. 10.1038/cddis.2017.314 - DOI - PMC - PubMed
-
- Colon-Cruz L, Rodriguez-Morales R, Santana-Cruz A, Cantres-Velez J, Torrado-Tapias A, Lin SJ, Yudowski G, Kensler R, Marie B, Burgess SM, Renaud O, Varshney GK, & Behra M (2021). Cnr2 Is Important for Ribbon Synapse Maturation and Function in Hair Cells and Photoreceptors. Front Mol Neurosci, 14, 624265. 10.3389/fnmol.2021.624265 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

