Coastal and deep-sea biodegradation of polyhydroxyalkanoate microbeads
- PMID: 38705904
- PMCID: PMC11070421
- DOI: 10.1038/s41598-024-60949-z
Coastal and deep-sea biodegradation of polyhydroxyalkanoate microbeads
Abstract
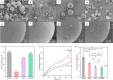
Microbeads find widespread usage in personal care items and cosmetics, serving as exfoliants or scrubbing agents. Their micro-scale size poses challenges in effective drainage capture and given their origin from non-biodegradable oil-based plastics, this contributes substantially to marine pollution. In this study, microbeads were prepared by a simple yet scalable melt homogenization method using four types of polyhydroxyalkanoates (PHA), namely poly[(R)-3-hydroxybutyrate] (P(3HB)), poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] (P(3HB-co-3HV)), poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] (P(3HB-co-3HHx)) and poly[(R)-3-hydroxybutyrate-co-(R)-4-hydroxyvalerate] (P(3HB-co-4HB)). Microbeads with different surface smoothness, compressive strength (6.2-13.3 MPa) and diameter (from 1 ~ 150 μm) could be produced. The microbeads were subjected to a comprehensive degradation analysis using three techniques: enzymatic, Biochemical Oxygen Demand (BOD) evaluations, and in situ degradation tests in the deep-sea off Misaki Port in the northern Pacific Ocean (depth of 757 m). Qualitatively, results from enzymatic and in situ degradation demonstrated significant degradation within one week and five months, respectively. Quantitatively, BOD findings indicated that all PHA microbeads degraded similarly to cellulose (~ 85% biodegradability in 25 days). In conclusion, PHA microbeads from this study exhibit promising potential as alternatives to conventional non-biodegradable microbeads.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Arthur, C., Baker, J. & Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris (2009).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

