Distinct neural markers of evidence accumulation index metacognitive processing before and after simple visual decisions
- PMID: 38706138
- PMCID: PMC11070453
- DOI: 10.1093/cercor/bhae179
Distinct neural markers of evidence accumulation index metacognitive processing before and after simple visual decisions
Abstract
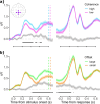
Perceptual decision-making is affected by uncertainty arising from the reliability of incoming sensory evidence (perceptual uncertainty) and the categorization of that evidence relative to a choice boundary (categorical uncertainty). Here, we investigated how these factors impact the temporal dynamics of evidence processing during decision-making and subsequent metacognitive judgments. Participants performed a motion discrimination task while electroencephalography was recorded. We manipulated perceptual uncertainty by varying motion coherence, and categorical uncertainty by varying the angular offset of motion signals relative to a criterion. After each trial, participants rated their desire to change their mind. High uncertainty impaired perceptual and metacognitive judgments and reduced the amplitude of the centro-parietal positivity, a neural marker of evidence accumulation. Coherence and offset affected the centro-parietal positivity at different time points, suggesting that perceptual and categorical uncertainty affect decision-making in sequential stages. Moreover, the centro-parietal positivity predicted participants' metacognitive judgments: larger predecisional centro-parietal positivity amplitude was associated with less desire to change one's mind, whereas larger postdecisional centro-parietal positivity amplitude was associated with greater desire to change one's mind, but only following errors. These findings reveal a dissociation between predecisional and postdecisional evidence processing, suggesting that the CPP tracks potentially distinct cognitive processes before and after a decision.
Keywords: centro-parietal positivity; decision-making; electroencephalography; metacognition; motion discrimination.
© The Author(s) 2024. Published by Oxford University Press.
Figures





References
-
- Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015:67(1):1–48. 10.18637/jss.v067.i01. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

