Quantitative ultrasound radiomics guided adaptive neoadjuvant chemotherapy in breast cancer: early results from a randomized feasibility study
- PMID: 38706611
- PMCID: PMC11066296
- DOI: 10.3389/fonc.2024.1273437
Quantitative ultrasound radiomics guided adaptive neoadjuvant chemotherapy in breast cancer: early results from a randomized feasibility study
Abstract
Background: In patients with locally advanced breast cancer (LABC) receiving neoadjuvant chemotherapy (NAC), quantitative ultrasound (QUS) radiomics can predict final responses early within 4 of 16-18 weeks of treatment. The current study was planned to study the feasibility of a QUS-radiomics model-guided adaptive chemotherapy.
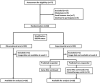
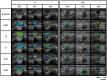
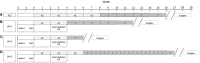
Methods: The phase 2 open-label randomized controlled trial included patients with LABC planned for NAC. Patients were randomly allocated in 1:1 ratio to a standard arm or experimental arm stratified by hormonal receptor status. All patients were planned for standard anthracycline and taxane-based NAC as decided by their medical oncologist. Patients underwent QUS imaging using a clinical ultrasound device before the initiation of NAC and after the 1st and 4th weeks of treatment. A support vector machine-based radiomics model developed from an earlier cohort of patients was used to predict treatment response at the 4th week of NAC. In the standard arm, patients continued to receive planned chemotherapy with the treating oncologists blinded to results. In the experimental arm, the QUS-based prediction was conveyed to the responsible oncologist, and any changes to the planned chemotherapy for predicted non-responders were made by the responsible oncologist. All patients underwent surgery following NAC, and the final response was evaluated based on histopathological examination.
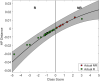
Results: Between June 2018 and July 2021, 60 patients were accrued in the study arm, with 28 patients in each arm available for final analysis. In patients without a change in chemotherapy regimen (53 of 56 patients total), the QUS-radiomics model at week 4 of NAC that was used demonstrated an accuracy of 97%, respectively, in predicting the final treatment response. Seven patients were predicted to be non-responders (observational arm (n=2), experimental arm (n=5)). Three of 5 non-responders in the experimental arm had chemotherapy regimens adapted with an early initiation of taxane therapy or chemotherapy intensification, or early surgery and ended up as responders on final evaluation.
Conclusion: The study demonstrates the feasibility of QUS-radiomics adapted guided NAC for patients with breast cancer. The ability of a QUS-based model in the early prediction of treatment response was prospectively validated in the current study.
Clinical trial registration: clinicaltrials.gov, ID NCT04050228.
Keywords: adaptive chemotherapy; artificial intelligence; breast cancer; neoadjuvant chemotherapy; quantitative ultrasound (QUS); radiomics.
Copyright © 2024 Dasgupta, DiCenzo, Sannachi, Gandhi, Pezo, Eisen, Warner, Wright, Look-Hong, Sadeghi-Naini, Curpen, Kolios, Trudeau and Czarnota.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Quantitative ultrasound radiomics in predicting response to neoadjuvant chemotherapy in patients with locally advanced breast cancer: Results from multi-institutional study.Cancer Med. 2020 Aug;9(16):5798-5806. doi: 10.1002/cam4.3255. Epub 2020 Jun 29. Cancer Med. 2020. PMID: 32602222 Free PMC article.
-
Quantitative ultrasound radiomics using texture derivatives in prediction of treatment response to neo-adjuvant chemotherapy for locally advanced breast cancer.Oncotarget. 2020 Oct 20;11(42):3782-3792. doi: 10.18632/oncotarget.27742. eCollection 2020 Oct 20. Oncotarget. 2020. PMID: 33144919 Free PMC article.
-
Early Changes in Quantitative Ultrasound Imaging Parameters during Neoadjuvant Chemotherapy to Predict Recurrence in Patients with Locally Advanced Breast Cancer.Cancers (Basel). 2022 Feb 28;14(5):1247. doi: 10.3390/cancers14051247. Cancers (Basel). 2022. PMID: 35267555 Free PMC article.
-
Implementation of Non-Invasive Quantitative Ultrasound in Clinical Cancer Imaging.Cancers (Basel). 2022 Dec 16;14(24):6217. doi: 10.3390/cancers14246217. Cancers (Basel). 2022. PMID: 36551702 Free PMC article. Review.
-
A Narrative Review of Ultrasound Technologies for the Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer.Cancer Manag Res. 2021 Oct 14;13:7885-7895. doi: 10.2147/CMAR.S331665. eCollection 2021. Cancer Manag Res. 2021. PMID: 34703310 Free PMC article. Review.
Cited by
-
Predicting Pathological Complete Response Following Neoadjuvant Therapy in Patients With Breast Cancer: Development of Machine Learning-Based Prediction Models in a Retrospective Study.JMIR Cancer. 2025 Jul 18;11:e64685. doi: 10.2196/64685. JMIR Cancer. 2025. PMID: 40680158 Free PMC article.
-
Harnessing Artificial Intelligence to Enhance Global Breast Cancer Care: A Scoping Review of Applications, Outcomes, and Challenges.Cancers (Basel). 2025 Jan 9;17(2):197. doi: 10.3390/cancers17020197. Cancers (Basel). 2025. PMID: 39857979 Free PMC article. Review.
-
Validation of a Quantitative Ultrasound Texture Analysis Model for Early Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer: A Prospective Serial Imaging Study.Cancers (Basel). 2025 Aug 7;17(15):2594. doi: 10.3390/cancers17152594. Cancers (Basel). 2025. PMID: 40805289 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

