Functional diversification of innate and inflammatory immune responses mediated by antibody fragment crystallizable activities against SARS-CoV-2
- PMID: 38706870
- PMCID: PMC11068556
- DOI: 10.1016/j.isci.2024.109703
Functional diversification of innate and inflammatory immune responses mediated by antibody fragment crystallizable activities against SARS-CoV-2
Abstract
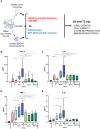
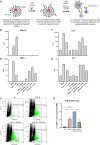
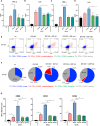
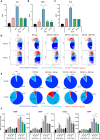
Monoclonal antibodies (mAb) targeting the SARS-CoV-2 Spike (S) glycoprotein have been exploited for the treatment of severe COVID-19. In this study, we evaluated the immune-regulatory features of two neutralizing anti-S mAbs (nAbs), named J08 and F05, with wild-type (WT) conformation or silenced Fc functions. In the presence of D614G SARS-CoV-2, WT nAbs enhance intracellular viral uptake in immune cells and amplify antiviral type I Interferon and inflammatory cytokine and chemokine production without viral replication, promoting the differentiation of CD16+ inflammatory monocytes and innate/adaptive PD-L1+ and PD-L1+CD80+ plasmacytoid Dendritic Cells. In spite of a reduced neutralizing property, WT J08 nAb still promotes the IL-6 production and differentiation of CD16+ monocytes once binding Omicron BA.1 variant. Fc-mediated regulation of antiviral and inflammatory responses, in the absence of viral replication, highlighted in this study, might positively tune immune response during SARS-CoV-2 infection and be exploited also in mAb-based therapeutic and prophylactic strategies against viral infections.
Keywords: Immune response; Immunology; Virology.
© 2024 The Authors. Published by Elsevier Inc.
Conflict of interest statement
SM, EMP, RD, CG, Canitano A, Cara A, FS, SP, PAT, and CEM declare no conflict of interest. AE and RR are listed as inventors of full-length human monoclonal antibodies described in Italian patent applications n. 102020000015754 filed on June 30th 2020, 102020000018955 filed on August 3rd 2020 and 102020000029969 filed on 4th of December 2020, and the international patent system number PCT/IB2021/055755 filed on the 28th of June 2021. All patents were submitted by Fondazione Toscana Life Sciences, Siena, Italy.
Figures

Similar articles
-
Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis.Microbiol Spectr. 2022 Aug 31;10(4):e0092622. doi: 10.1128/spectrum.00926-22. Epub 2022 Jun 14. Microbiol Spectr. 2022. PMID: 35700134 Free PMC article.
-
Molecular Characterization of AZD7442 (Tixagevimab-Cilgavimab) Neutralization of SARS-CoV-2 Omicron Subvariants.Microbiol Spectr. 2023 Mar 6;11(2):e0033323. doi: 10.1128/spectrum.00333-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 36877050 Free PMC article.
-
Antibody-dependent NK-cell and neutralizing antibody responses against the Spike protein of Wuhan-Hu-1 and Omicron BA.1 SARS-CoV-2 variants in vaccinated experienced and vaccinated naïve individuals.J Med Virol. 2023 Jul;95(7):e28900. doi: 10.1002/jmv.28900. J Med Virol. 2023. PMID: 37403730
-
Identification and Analysis of Monoclonal Antibodies with Neutralizing Activity against Diverse SARS-CoV-2 Variants.J Virol. 2023 Jun 29;97(6):e0028623. doi: 10.1128/jvi.00286-23. Epub 2023 May 16. J Virol. 2023. PMID: 37191569 Free PMC article.
-
Status and Developing Strategies for Neutralizing Monoclonal Antibody Therapy in the Omicron Era of COVID-19.Viruses. 2023 May 31;15(6):1297. doi: 10.3390/v15061297. Viruses. 2023. PMID: 37376597 Free PMC article. Review.
Cited by
-
Simian Immunodeficiency Virus-Based Virus-like Particles Are an Efficient Tool to Induce Persistent Anti-SARS-CoV-2 Spike Neutralizing Antibodies and Specific T Cells in Mice.Vaccines (Basel). 2025 Feb 21;13(3):216. doi: 10.3390/vaccines13030216. Vaccines (Basel). 2025. PMID: 40266067 Free PMC article.
-
Flow cytometric procedures for deep characterization of nanoparticles.Biol Methods Protoc. 2025 Mar 11;10(1):bpaf019. doi: 10.1093/biomethods/bpaf019. eCollection 2025. Biol Methods Protoc. 2025. PMID: 40160935 Free PMC article.
References
-
- Hirsch C., Park Y.S., Piechotta V., Chai K.L., Estcourt L.J., Monsef I., Salomon S., Wood E.M., So-Osman C., McQuilten Z., et al. SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19. Cochrane Database Syst. Rev. 2022;6:CD014945. doi: 10.1002/14651858.CD014945.pub2. - DOI - PMC - PubMed
-
- Savoldi A., Morra M., De Nardo P., Cattelan A.M., Mirandola M., Manfrin V., Scotton P., Giordani M.T., Brollo L., Panese S., et al. Clinical efficacy of different monoclonal antibody regimens among non-hospitalised patients with mild to moderate COVID-19 at high risk for disease progression: a prospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2022;41:1065–1076. doi: 10.1007/s10096-022-04464-x. - DOI - PMC - PubMed
-
- Savoldi A., Morra M., Castelli A., Mirandola M., Berkell M., Smet M., Konnova A., Rossi E., Cataudella S., De Nardo P., et al. Clinical Impact of Monoclonal Antibodies in the Treatment of High-Risk Patients with SARS-CoV-2 Breakthrough Infections: The ORCHESTRA Prospective Cohort Study. Biomedicines. 2022;10 doi: 10.3390/biomedicines10092063. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

