A Longitudinal Analysis of Black Box Warnings: Trends and Implications for Drug Safety
- PMID: 38706997
- PMCID: PMC11069364
- DOI: 10.7759/cureus.57597
A Longitudinal Analysis of Black Box Warnings: Trends and Implications for Drug Safety
Abstract
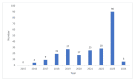
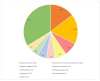
A black box warning, signaling potential life-threatening adverse effects of medications or medical devices, is crucial for public and healthcare professional awareness. Comprehending and adhering to these warnings can prevent serious harm. This review aims to elucidate their significance. Data on drugs with black box warnings were collected from the Food and Drug Administration's (FDA's) official website using the search term 'Boxed warnings' from January 1, 2015, to January 31, 2024. A Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA) containing black box warnings for this period was downloaded from the FDA's website. Additional parameters, such as drug class and whether the warnings were new or existing, were added to the downloaded spreadsheet. The collected data were organized by year, categorizing new and existing warnings, along with details on the evidence source, system-wise classification, and black box warnings for commonly used drugs, including their clinical significance. Results show that in the past decade, 40% of black box warnings were issued in 2023, followed by 12% in 2022. Most warnings (67%) comprised existing ones with minor revisions while 29% were new. Nine existing warnings were removed during the period. Post-marketing studies predominantly provided evidence for these warnings. Neuropsychiatric concerns like addiction potential (31%), suicidal tendency (7%), and hypersensitivity reactions (12%) were the frequently encountered black box warnings. Black box warnings play a crucial role in highlighting the serious adverse effects of medications. Neuropsychiatric warnings have been frequent over the past decade. Awareness of these warnings is essential to prevent adverse effects and enhance patient care, especially concerning drugs like guaifenesin/hydrocodone bitartrate, zolpidem, and montelukast commonly encountered in clinical practice.
Keywords: adverse events (ae); black box warnings; drug label; drugs; post-marketing studies.
Copyright © 2024, Rajendran et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Medicare Prescription Drug Plan Formulary Restrictions After Postmarket FDA Black Box Warnings.J Manag Care Spec Pharm. 2019 Nov;25(11):1201-1217. doi: 10.18553/jmcp.2019.25.11.1201. J Manag Care Spec Pharm. 2019. PMID: 31663461 Free PMC article.
-
Impact of United States Food and Drug Administration's boxed warnings on adverse drug reactions reporting rates and risk mitigation for multiple myeloma drugs.Expert Opin Drug Saf. 2013 May;12(3):299-307. doi: 10.1517/14740338.2013.780024. Epub 2013 Mar 12. Expert Opin Drug Saf. 2013. PMID: 23480866
-
Risk management policy and black-box warnings: a qualitative analysis of US FDA proceedings.Drug Saf. 2009;32(11):1057-66. doi: 10.2165/11316670-000000000-00000. Drug Saf. 2009. PMID: 19810777
-
FDA boxed warnings: how to prescribe drugs safely.Am Fam Physician. 2010 Feb 1;81(3):298-303. Am Fam Physician. 2010. PMID: 20112888 Review.
-
Black-Box Warnings of Antiseizure Medications: What is Inside the Box?Pharmaceut Med. 2023 May;37(3):233-250. doi: 10.1007/s40290-023-00475-x. Epub 2023 Apr 29. Pharmaceut Med. 2023. PMID: 37119452 Review.
References
-
- Black box warnings. [ Mar; 2024 ]. 2023. https://www.drugwatch.com/fda/black-box-warnings/#sources https://www.drugwatch.com/fda/black-box-warnings/#sources
-
- Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products — content and format. [ Mar; 2024 ]. 2011. https://www.fda.gov/regulatory-information/search-fda-guidance-documents... https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- "Black box" 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. Murphy S, Roberts R. J Allergy Clin Immunol. 2006;117:34–39. - PubMed
-
- CFR - Code of Federal Regulations Title 21. [ Mar; 2024 ]. 2023. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?C... https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?C...
-
- Ethical and practical considerations in removing black box warnings from drug labels. Yeh JS, Sarpatwari A, Kesselheim AS. Drug Saf. 2016;39:709–714. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
