Abemaciclib-Associated Skin, Hair, and Nail Toxicities: A Case Report
- PMID: 38707105
- PMCID: PMC11070195
- DOI: 10.7759/cureus.57677
Abemaciclib-Associated Skin, Hair, and Nail Toxicities: A Case Report
Abstract
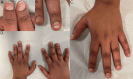
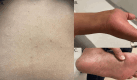
Abemaciclib is a cyclin-dependent kinase 4/6 inhibitor that is utilized to manage hormone-sensitive human epidermal growth factor receptor-2 positive metastatic breast cancer. Palbociclib and ribociclib are types of other orally administered CDK4/6i that share similar safety and tolerability compared with Abemaciclib. Reported side effects include reversible neutropenia of a lower grade, gastrointestinal toxicity, and anemia. CDK4/6i could induce dermatological side effects. Although less frequent, cutaneous adverse effects of CDK4/6i account for 25% of medication discontinuation. Frequent cutaneous adverse events are rash and pruritus; nonetheless, hair loss, nail changes, vitiligo, and photosensitivity reactions, were also reported to a lesser extent. Herein, we report a case of hair, nail, and pigmentary disorder that occurred 10 months after initiating abemaciclib treatment.
Keywords: abemaciclib; cdk4/6i; cyclin-dependent kinase 4/6 inhibitors; palbociclib; ribociclib.
Copyright © 2024, Alsatti et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
CDK4/6 Inhibitors Expand the Therapeutic Options in Breast Cancer: Palbociclib, Ribociclib and Abemaciclib.BioDrugs. 2019 Apr;33(2):125-135. doi: 10.1007/s40259-019-00337-6. BioDrugs. 2019. PMID: 30847853 Review.
-
Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations.Oncologist. 2017 Sep;22(9):1039-1048. doi: 10.1634/theoncologist.2017-0142. Epub 2017 Jul 13. Oncologist. 2017. PMID: 28706010 Free PMC article. Review.
-
Healthcare Resource Utilization and Cost Comparison Between Palbociclib, Abemaciclib, and Ribociclib Among Patients with HR+/HER2- Metastatic Breast Cancer.Clinicoecon Outcomes Res. 2025 Mar 26;17:247-264. doi: 10.2147/CEOR.S496100. eCollection 2025. Clinicoecon Outcomes Res. 2025. PMID: 40165979 Free PMC article.
-
Real world incidence and management of adverse events in patients with HR+, HER2- metastatic breast cancer receiving CDK4 and 6 inhibitors in a United States community setting.Curr Med Res Opin. 2022 Aug;38(8):1319-1331. doi: 10.1080/03007995.2022.2073122. Epub 2022 May 13. Curr Med Res Opin. 2022. PMID: 35535675
-
Real-World Patient Characteristics, Utilization Patterns, and Outcomes of US Patients with HR+, HER2- Metastatic Breast Cancer Treated with Abemaciclib.Drugs Real World Outcomes. 2022 Dec;9(4):681-693. doi: 10.1007/s40801-022-00327-1. Epub 2022 Sep 12. Drugs Real World Outcomes. 2022. PMID: 36097254 Free PMC article.
Cited by
-
Abemaciclib-Associated Panniculitis With Fibrosis: Expanding the Dermatologic Spectrum of Cyclin-Dependent Kinase 4/6 Inhibitors.Cureus. 2025 May 2;17(5):e83380. doi: 10.7759/cureus.83380. eCollection 2025 May. Cureus. 2025. PMID: 40458320 Free PMC article.
References
-
- Comparison of treatment-related adverse events of different cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: a network meta-analysis. Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Cancer Treat Rev. 2020;90:102086. - PubMed
-
- Skin toxicities with cyclin-dependent kinase 4/6 inhibitors in breast cancer: signals from disproportionality analysis of the FDA Adverse Event Reporting System. Raschi E, Fusaroli M, La Placa M, Ardizzoni A, Zamagni C, Poluzzi E, De Ponti F. Am J Clin Dermatol. 2022;23:247–255. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
