Green and sustainable use of macadamia nuts as support material in Pt-based direct methanol fuel cells
- PMID: 38707303
- PMCID: PMC11068541
- DOI: 10.1016/j.heliyon.2024.e29907
Green and sustainable use of macadamia nuts as support material in Pt-based direct methanol fuel cells
Abstract
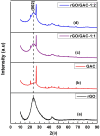
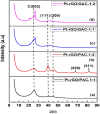
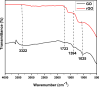
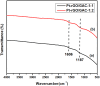
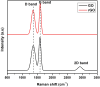
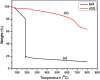
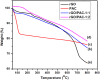
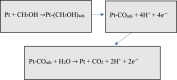
The successful commercialization of direct methanol fuel cells (DMFCs) is hindered by inadequate methanol oxidation activity and anode catalyst longevity. Efficient and cost-effective electrode materials are imperative in the widespread use of DMFCs. While Platinum (Pt) remains the primary component of anodic methanol oxidation reaction (MOR) electrocatalysts, its utilization alone in DMFC systems is limited due to carbon monoxide (CO) poisoning, instability, methanol crossover, and high cost. These limitations impede the economic feasibility of Pt as an electrocatalyst. Herein, we present the use of powdered activated carbon (PAC) and granular activated carbon (GAC), both sourced from macadamia nut shells (MNS), a type of biomass. These bio-based carbon materials are integrated into hybrid supports with reduced graphene oxide (rGO), aiming to enhance the performance and reduce the production cost of the Pt electrocatalyst. Electrochemical and physicochemical characterizations of the synthesized catalysts, including Pt-rGO/PAC-1:1, Pt-rGO/PAC-1:2, Pt-rGO/GAC-1:1, and Pt-rGO/GAC-1:2, were conducted. X-ray diffraction analysis revealed crystallite sizes ranging from 1.18 nm to 1.68 nm. High-resolution transmission electron microscopy (HRTEM) images with average particle sizes ranging from 1.91 nm to 2.72 nm demonstrated spherical dispersion of Pt nanoparticles with some agglomeration across all catalysts. The electrochemical active surface area (ECSA) was determined, with Pt-rGO/GAC-1:1 exhibiting the highest ECSA of 73.53 m2 g-1. Despite its high ECSA, Pt-rGO/GAC-1:1 displayed the lowest methanol oxidation reaction (MOR) current density, indicating active sites with poor catalytic efficiency. Pt-rGO/PAC-1:1 and Pt-rGO/PAC-1:2 exhibited the highest MOR current densities of 0.77 mA*cm-2 and 0.74 mA*cm-2, respectively. Moreover, Pt-rGO/PAC-1:2 and Pt-rGO/PAC-1:1 demonstrated superior electrocatalytic mass (specific) activities of 7.55 mA/mg (0.025 mA*cm-2) and 7.25 mA/mg (0.021 mA*cm-2), respectively. Chronoamperometry tests revealed Pt-rGO/PAC-1:2 and Pt-rGO/PAC-1:1 as the most stable catalysts. Additionally, they exhibited the lowest charge transfer resistances and highest MOR current densities after durability tests, highlighting their potential for DMFC applications. The synthesized Pt supported on PACs hybrids demonstrated remarkable catalytic performance, stability, and CO tolerance, highlighting their potential for enhancing DMFC efficiency.
Keywords: Biowaste; Direct methanol fuel cell; Green chemistry; Macadamia nuts; Pt electrocatalysts.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures























References
-
- Kurniawan T.A., Othman M.H.D., Liang X., Goh H.H., Gikas P., Kusworo T.D., Anouzla A., Chew K.W. Decarbonization in waste recycling industry using digitalization to promote net-zero emissions and its implications on sustainability. J. Environ. Manag. 2023;338 - PubMed
-
- Yusaf T., Mahamude A.S.F., Kadirgama K., Ramasamy D., Farhana K., Dhahad H.A., Talib A.R.A. Sustainable hydrogen energy in aviation–A narrative review. Int. J. Hydrogen Energy. 2023;52:1026–1045.
-
- Aithal S., Aithal P.S. Nanotechnology based mega machine design for large scale air cleaning–prospects and challenges. Int. J. Case Stud. Bus. IT, Educ. (IJCSBE) 2020;4(2):250–269.
-
- Wang Y., Tian W., Wan J., Zheng Y., Zhang H., Wang Y. Tuning coordination microenvironment of V2CTx MXene for anchoring single-atom toward efficient multifunctional electrocatalysis. J. Colloid Interface Sci. 2023;645:833–840. - PubMed
-
- Abdullah-Al-Mahbub M., Islam A.R.M.T., Almohamad H., Al Dughairi A.A., Al-Mutiry M., Abdo H.G. Different forms of solar energy progress: the fast-growing eco-friendly energy source in Bangladesh for a sustainable future. Energies. 2022;15(18):6790.
LinkOut - more resources
Full Text Sources

