Qufeng tongluo decoction decreased proteinuria in diabetic mice by protecting podocytes via promoting autophagy
- PMID: 38707926
- PMCID: PMC11068988
- DOI: 10.1016/j.jtcme.2023.11.007
Qufeng tongluo decoction decreased proteinuria in diabetic mice by protecting podocytes via promoting autophagy
Abstract
Background: Diabetic kidney disease (DKD) is one of diabetic complications, which has become the leading cause of end-stage kidney disease. In addition to angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker(ACEI/ARB) and sodium-glucose cotransporter-2 inhibitor (SGLT2i), traditional Chinese medicine (TCM) is an effective alternative treatment for DKD. In this study, the effect of Qufeng Tongluo (QFTL) decoction in decreasing proteinuria has been observed and its mechanism has been explored based on autophagy regulation in podocyte.
Methods: In vivo study, db/db mice were used as diabetes model and db/m mice as blank control. Db/db mice were treated with QFTL decoction, rapamycin, QFTL + 3-Methyladenine (3-MA), trehalose, chloroquine (CQ) and QFTL + CQ. Mice urinary albumin/creatinine (UACR), nephrin and autophagy related proteins (LC3 and p62) in kidney tissue were detected after intervention of 9 weeks. Transcriptomics was operated with the kidney tissue from model group and QFTL group. In vitro study, mouse podocyte clone-5 (MPC-5) cells were stimulated with hyperglycemic media (30 mmol/L glucose) or cultured with normal media. High-glucose-stimulated MPC-5 cells were treated with QFTL freeze-drying powder, rapamycin, CQ, trehalose, QFTL+3-MA and QFTL + CQ. Cytoskeletal actin, nephrin, ATG-5, ATG-7, Beclin-1, cathepsin L and cathepsin B were assessed. mRFP-GFP-LC3 was established by stubRFP-sensGFP-LC3 lentivirus transfection.
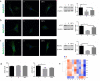
Results: QFTL decoction decreased the UACR and increased the nephrin level in kidney tissue and high-glucose-stimulated podocytes. Autophagy inhibitors, including 3-MA and chloroquine blocked the effects of QFTL decoction. Further study showed that QFTL decoction increased the LC3 expression and relieved p62 accumulation in podocytes of db/db mice. In high-glucose-stimulated MPC-5 cells, QFTL decoction rescued the inhibited LC3 and promoted the expression of ATG-5, ATG-7, and Beclin-1, while had no effect on the activity of cathepsin L and cathepsin B. Results of transcriptomics also showed that 51 autophagy related genes were regulated by QFTL decoction, including the genes of ATG10, SCOC, ATG4C, AMPK catalytic subunit, PI3K catalytic subunit, ATG3 and DRAM2.
Conclusion: QFTL decoction decreased proteinuria and protected podocytes in db/db mice by regulating autophagy.
Keywords: Autophagy; Podocyte; Proteinuria; Qufeng tongluo decoction; Traditional Chinese medicine.
© 2023 Center for Food and Biomolecules, National Taiwan University. Production and hosting by Elsevier Taiwan LLC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Tongluo Digui decoction treats renal injury in diabetic rats by promoting autophagy of podocytes.J Tradit Chin Med. 2021 Feb;41(1):125-132. doi: 10.19852/j.cnki.jtcm.2021.01.014. J Tradit Chin Med. 2021. PMID: 33522205
-
Jiedu Tongluo Baoshen formula enhances podocyte autophagy and reduces proteinuria in diabetic kidney disease by inhibiting PI3K/Akt/mTOR signaling pathway.J Ethnopharmacol. 2022 Jul 15;293:115246. doi: 10.1016/j.jep.2022.115246. Epub 2022 Apr 7. J Ethnopharmacol. 2022. PMID: 35398500
-
Qizhi Jiangtang capsule activates podocyte autophagy in diabetic kidney disease by inhibiting phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathways.J Tradit Chin Med. 2023 Aug;43(4):667-675. doi: 10.19852/j.cnki.jtcm.20230428.001. J Tradit Chin Med. 2023. PMID: 37454251 Free PMC article.
-
Heme oxygenase-1 enhances autophagy in podocytes as a protective mechanism against high glucose-induced apoptosis.Exp Cell Res. 2015 Oct 1;337(2):146-59. doi: 10.1016/j.yexcr.2015.04.005. Epub 2015 Apr 13. Exp Cell Res. 2015. PMID: 25882498 Review.
-
mTOR-mediated nutrient sensing and oxidative stress pathways regulate autophagy: a key mechanism for traditional Chinese medicine to improve diabetic kidney disease.Front Pharmacol. 2025 Apr 23;16:1578400. doi: 10.3389/fphar.2025.1578400. eCollection 2025. Front Pharmacol. 2025. PMID: 40337513 Free PMC article. Review.
Cited by
-
Mechanisms of Xuefu Zhuyu decoction in treating diabetic kidney disease-induced renal fibrosis: UPLC-Q/TOF-MS, network pharmacology, and experimental validation.Korean J Physiol Pharmacol. 2025 Sep 1;29(5):571-597. doi: 10.4196/kjpp.24.330. Epub 2025 Jul 28. Korean J Physiol Pharmacol. 2025. PMID: 40717511 Free PMC article.
-
Novel Insights into Diabetic Kidney Disease.Int J Mol Sci. 2024 Sep 23;25(18):10222. doi: 10.3390/ijms251810222. Int J Mol Sci. 2024. PMID: 39337706 Free PMC article. Review.
References
-
- Gill H.K., Kaur P., Mahendru S., Mithal A. Adverse effect profile and effectiveness of sodium glucose Co-transporter 2 inhibitors (SGLT2i) - a prospective real-world setting study. Indian journal of endocrinology and metabolism. Jan-Feb 2019;23(1):50–55. doi: 10.4103/ijem.IJEM_566_18. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
