Beyond Immune Balance: The Pivotal Role of Decidual Regulatory T Cells in Unexplained Recurrent Spontaneous Abortion
- PMID: 38707955
- PMCID: PMC11070170
- DOI: 10.2147/JIR.S459263
Beyond Immune Balance: The Pivotal Role of Decidual Regulatory T Cells in Unexplained Recurrent Spontaneous Abortion
Abstract
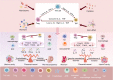
Recurrent spontaneous abortion (RSA) is defined as two or more consecutive pregnancy failures, which brings tremendous stress to women of childbearing age and seriously affects family well-being. However, the reason in about 50% of cases remains unknown and is defined as unexplained recurrent spontaneous abortion (URSA). The immunological perspective in URSA has attracted widespread attention in recent years. The embryo is regarded as a semi-allogeneic graft to the mother. A successful pregnancy requires transition to an immune environment conducive to embryo survival at the maternal-fetal interface. As an important member of regulatory immunity, regulatory T (Treg) cells play a key role in regulating immune tolerance at the maternal-fetal interface. This review will focus on the phenotypic plasticity and lineage stability of Treg cells to illustrate its relationship with URSA.
Keywords: Treg cells; immune homeostasis; maternal–fetal tolerance; phenotype.
© 2024 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion.Biosci Trends. 2018 May 13;12(2):157-167. doi: 10.5582/bst.2018.01012. Epub 2018 Apr 15. Biosci Trends. 2018. PMID: 29657243
-
The role of Th17 and Treg cells in normal pregnancy and unexplained recurrent spontaneous abortion (URSA): New insights into immune mechanisms.Placenta. 2023 Oct;142:18-26. doi: 10.1016/j.placenta.2023.08.065. Epub 2023 Aug 16. Placenta. 2023. PMID: 37603948 Review.
-
Reduced frequency and functional defects of CD4+CD25highCD127low/- regulatory T cells in patients with unexplained recurrent spontaneous abortion.Reprod Biol Endocrinol. 2020 Jun 10;18(1):62. doi: 10.1186/s12958-020-00619-7. Reprod Biol Endocrinol. 2020. PMID: 32522204 Free PMC article.
-
Polarization disorder of decidual NK cells in unexplained recurrent spontaneous abortion revealed by single-cell transcriptome analysis.Reprod Biol Endocrinol. 2022 Jul 27;20(1):108. doi: 10.1186/s12958-022-00980-9. Reprod Biol Endocrinol. 2022. PMID: 35897028 Free PMC article.
-
Maternal-fetal immunity and recurrent spontaneous abortion.Am J Reprod Immunol. 2024 May;91(5):e13859. doi: 10.1111/aji.13859. Am J Reprod Immunol. 2024. PMID: 38722063 Review.
Cited by
-
Extracellular vesicles from human semen induce unique tolerogenic phenotypes in vaginal dendritic cells and regulatory T lymphocytes.Front Immunol. 2025 May 12;16:1564002. doi: 10.3389/fimmu.2025.1564002. eCollection 2025. Front Immunol. 2025. PMID: 40421022 Free PMC article.
-
Exploring the role of endoplasmic reticulum stress in recurrent spontaneous abortion: Identification of diagnostic biomarkers and immune cell interactions.Heliyon. 2024 Oct 5;10(19):e38964. doi: 10.1016/j.heliyon.2024.e38964. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39430538 Free PMC article.
-
Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure.Int J Mol Sci. 2025 Feb 3;26(3):1295. doi: 10.3390/ijms26031295. Int J Mol Sci. 2025. PMID: 39941063 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

