Deep Electroreductive Chemistry: Harnessing Carbon- and Silicon-based Reactive Intermediates in Organic Synthesis
- PMID: 38707967
- PMCID: PMC11067979
- DOI: 10.1021/acscatal.3c01174
Deep Electroreductive Chemistry: Harnessing Carbon- and Silicon-based Reactive Intermediates in Organic Synthesis
Abstract
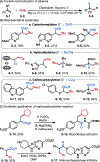
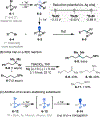
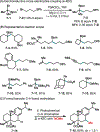
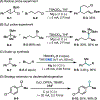
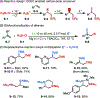
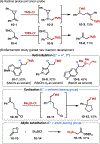
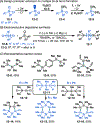
This Viewpoint outlines our recent contribution in electroreductive synthesis. Specifically, we leveraged deeply reducing potentials provided by electrochemistry to generate radical and anionic intermediates from readily available alkyl halides and chlorosilanes. Harnessing the distinct reactivities of radicals and anions, we have achieved several challenging transformations to construct C-C, C-Si, and Si-Si bonds. We highlight the mechanistic design principle that underpinned the development of each transformation and provide a view forward on future opportunities in growing area of reductive electrosynthesis.
Keywords: alkene difunctionalization; alkyl halide; chlorosilane; cross-electrophile coupling; electroreduction; electrosynthesis; radical-polar crossover.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Electroreductive Cross-Coupling Reactions: Carboxylation, Deuteration, and Alkylation.Acc Chem Res. 2025 Jan 7;58(1):113-129. doi: 10.1021/acs.accounts.4c00652. Epub 2024 Dec 13. Acc Chem Res. 2025. PMID: 39670841
-
An Electroreductive Approach to Radical Silylation via the Activation of Strong Si-Cl Bond.J Am Chem Soc. 2020 Dec 23;142(51):21272-21278. doi: 10.1021/jacs.0c10899. Epub 2020 Dec 8. J Am Chem Soc. 2020. PMID: 33290654 Free PMC article.
-
Recent Advances in First-Row Transition Metal-Catalyzed Reductive Coupling Reactions for π-Bond Functionalization and C-Glycosylation.Acc Chem Res. 2023 Nov 21;56(22):3292-3312. doi: 10.1021/acs.accounts.3c00531. Epub 2023 Nov 2. Acc Chem Res. 2023. PMID: 37917928
-
Electroreductive Cross-Electrophile Coupling (eXEC) Reactions.Angew Chem Int Ed Engl. 2023 Nov 6;62(45):e202306679. doi: 10.1002/anie.202306679. Epub 2023 Jul 12. Angew Chem Int Ed Engl. 2023. PMID: 37327185 Review.
-
Electroreductively Induced Radicals for Organic Synthesis.Molecules. 2023 Jan 15;28(2):857. doi: 10.3390/molecules28020857. Molecules. 2023. PMID: 36677915 Free PMC article. Review.
Cited by
-
Three-Component Cross-Electrophile Coupling: Regioselective Electrochemical Dialkylation of Alkenes.J Am Chem Soc. 2023 Oct 18;145(41):22298-22304. doi: 10.1021/jacs.3c06794. Epub 2023 Oct 6. J Am Chem Soc. 2023. PMID: 37801465 Free PMC article.
-
Electroreductive Radical Addition-Polar Cyclization Cascade to Access Cycloalkanes.Org Lett. 2024 Jan 12;26(1):116-121. doi: 10.1021/acs.orglett.3c03722. Epub 2023 Dec 29. Org Lett. 2024. PMID: 38157449 Free PMC article.
-
Facile, general allylation of unactivated alkyl halides via electrochemically enabled radical-polar crossover.Chem Sci. 2025 Mar 12;16(15):6317-6324. doi: 10.1039/d4sc07923j. eCollection 2025 Apr 9. Chem Sci. 2025. PMID: 40083972 Free PMC article.
-
The role of silicon in drug discovery: a review.RSC Med Chem. 2024 Jul 1;15(10):3286-3344. doi: 10.1039/d4md00169a. eCollection 2024 Oct 17. RSC Med Chem. 2024. PMID: 39430101 Free PMC article. Review.
-
Switchable electrochemical pathways for the selective C(sp3)-Ge bond formation.Nat Commun. 2025 Aug 6;16(1):7247. doi: 10.1038/s41467-025-62141-x. Nat Commun. 2025. PMID: 40770175 Free PMC article.
References
-
- Moss RA; Platz MS; Jones M Jr. Reactive Intermediate Chemistry, Wiley-VCH, Weinheim, 2015.
-
- Hartwig J. Organotransition Metal Chemistry: From Bonding to Catalysis. University Science Books, New York, 2010
-
- List B. Introduction: Organocatalysis. Chem. Rev 2007, 107, 5413–5415.
- MacMillan DWC The advent and development of organocatalysis. Nature 2008, 455, 304–308. - PubMed
-
- Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322–5363. - PMC - PubMed
- Narayanam JMR; Stephenson CRJ Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev 2011, 40, 102–113. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
