Practical tool to identify Spasticity-Plus Syndrome amongst patients with multiple sclerosis. Algorithm development based on a conjoint analysis
- PMID: 38708001
- PMCID: PMC11066270
- DOI: 10.3389/fneur.2024.1371644
Practical tool to identify Spasticity-Plus Syndrome amongst patients with multiple sclerosis. Algorithm development based on a conjoint analysis
Abstract
Introduction: The Spasticity-Plus Syndrome (SPS) in multiple sclerosis (MS) refers to a combination of spasticity and other signs/symptoms such as spasms, cramps, bladder dysfunction, tremor, sleep disorder, pain, and fatigue. The main purpose is to develop a user-friendly tool that could help neurologists to detect SPS in MS patients as soon as possible.
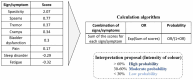
Methods: A survey research based on a conjoint analysis approach was used. An orthogonal factorial design was employed to form 12 patient profiles combining, at random, the eight principal SPS signs/symptoms. Expert neurologists evaluated in a survey and a logistic regression model determined the weight of each SPS sign/symptom, classifying profiles as SPS or not.
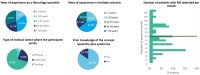
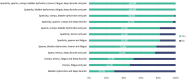
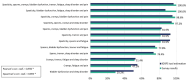
Results: 72 neurologists participated in the survey answering the conjoint exercise. Logistic regression results of the survey showed the relative contribution of each sign/symptom to the classification as SPS. Spasticity was the most influential sign, followed by spasms, tremor, cramps, and bladder dysfunction. The goodness of fit of the model was appropriate (AUC = 0.816). Concordance between the experts' evaluation vs. model estimation showed strong Pearson's (r = 0.936) and Spearman's (r = 0.893) correlation coefficients. The application of the algorithm provides with a probability of showing SPS and the following ranges are proposed to interpret the results: high (> 60%), moderate (30-60%), or low (< 30%) probability of SPS.
Discussion: This study offers an algorithmic tool to help healthcare professionals to identify SPS in MS patients. The use of this tool could simplify the management of SPS, reducing side effects related with polypharmacotherapy.
Keywords: Spasticity-Plus Syndrome; bladder dysfunction; conjoint analysis; multiple sclerosis; nabiximols; spasticity.
Copyright © 2024 Fernández Fernández, Costa-Frossard, Martínez Ginés, Montero Escribano, Prieto González, Ramió-Torrentà, Aladro, Alonso Torres, Álvarez Rodríguez, Labiano-Fontcuberta, Landete Pascual, Miralles Martínez, Moral Torres and Oliva-Nacarino.
Conflict of interest statement
ÓF has received honoraria in the past as consultant in advisory boards, and as chairmen or lecturer in meetings, and has also participated or participates at present in clinical trials and other research projects promoted by Biogen, Bayer, Merck-Serono, Teva, Novartis, Actelion, Almirall, Roche, Allergan, Horizon, and Ala Diagnostics. LC-F has served at scientific advisory boards, participated in meetings sponsored by and received speaking honoraria or travel funding or research grants from Biogen, Bristol-Myers Squibb, Janssen, Horizon, Merck-Serono, Novartis, Roche and Sanofi. MM has received honoraria as speaker, consultant in advisory boards, travel support or research grants from Merck, Biogen, Novartis, Sanofi-Genzyme, Almirall, Viatris, Horizon, Bristol-Myers Squibb, Roche, Sandoz and Janssen. PM has received compensation for consulting services and speaking fees from Allergan, Almirall, Biogen, Merz and Sanofi. JP has served as consultant, speaker and/or moderator for Bayer Pharmaceuticals, Biogen, Bristol-Myers Squibb, Daiichi Sankyo, Genzyme Corporation, Janssen, Merck Serono, Novartis, Sanofi, Sandoz, Teva, Roche Pharma, Almirall and Celgene. LR-T has received compensation for consulting services and speaking fees from Biogen, Novartis, Bayer, Merck, Sanofi, Genzyme, Roche, Bristol-Myers Squibb, Teva and Janssen. AM has received honoraria for scientific and commercial activities from Biogen, Teva, Merck, Sanofi, Novartis, Roche, Almirall, and Mylan. YA has received research grants, travel support and lecturing and consulting fees from Bayer, Biogen, Roche, Merck, Novartis, Almirall, Sanofi-Genzyme, Janssen and Bristol-Myers Squibb. AA has received honoraria as a speaker or advisor from Almirall, Biogen, Bristol-Myers Squibb, Janssen, Merck, Novartis, Roche and Sanofi. EÁ has received collaborations and fees for participation in advisories, scientific and educational activities from Almirall, Biogen, Janssen Cilag, Sanofi-Genzyme, Merck, Novartis, Roche and Teva. AL-F reports no disclosures relevant to the manuscript. LL has received collaborations and fees for participation in advisories, scientific and educational activities, from Almirall, Bayer, Biogen, Bristol-Myers Squibb, Sanofi-Genzyme, Merck, Novartis, UCB Pharma, Roche and Teva. EM has received honoraria as consultant in advisory boards, and or as chairperson or lecturer in meetings, attendance to congresses and has also participated in clinical trials and other research projects promoted by Almirall, Bayer, Biogen, Bristol Myers Squibb, Janssen, Merck, Teva, Novartis, Roche, Sandoz and Sanofi-Genzyme. PO-N has received collaborations and fees for participation in advisories, scientific and educational activities from Almirall, Biogen, Bristol-Myers Squibb, Sanofi-Genzyme, Merck, Novartis, UCB Pharma, Roche and Teva.
Figures
Similar articles
-
Spasticity-Plus syndrome in multiple sclerosis patients in a tertiary hospital in Spain.Front Neurol. 2024 Feb 26;15:1360032. doi: 10.3389/fneur.2024.1360032. eCollection 2024. Front Neurol. 2024. PMID: 38469589 Free PMC article.
-
Integrated Management of Multiple Sclerosis Spasticity and Associated Symptoms Using the Spasticity-Plus Syndrome Concept: Results of a Structured Specialists' Discussion Using the Workmat® Methodology.Front Neurol. 2021 Sep 27;12:722801. doi: 10.3389/fneur.2021.722801. eCollection 2021. Front Neurol. 2021. PMID: 34646229 Free PMC article.
-
Theoretical and Therapeutic Implications of the Spasticity-Plus Syndrome Model in Multiple Sclerosis.Front Neurol. 2022 Feb 7;12:802918. doi: 10.3389/fneur.2021.802918. eCollection 2021. Front Neurol. 2022. PMID: 35197915 Free PMC article.
-
Multiple sclerosis spasticity: 'state-of-the-art' questionnaire survey of specialized healthcare professionals.Expert Rev Neurother. 2013 Feb;13(3 Suppl 1):21-5. doi: 10.1586/ern.13.10. Expert Rev Neurother. 2013. PMID: 23369056 Review.
-
A systematic review of European regional and national guidelines: a focus on the recommended use of nabiximols in the management of spasticity in multiple sclerosis.Expert Rev Neurother. 2022 Jun;22(6):499-511. doi: 10.1080/14737175.2022.2075263. Epub 2022 Jun 1. Expert Rev Neurother. 2022. PMID: 35582858
References
-
- The British Medical Association (2002). Illustrated Medical Dictionary. London: Dorling Kindersley, 177–536
-
- Fernández Ó, Costa-Frossard L, Martínez-Ginés M, Montero P, Prieto JM, Ramió L. The broad concept of “spasticity-plus syndrome” in multiple sclerosis: a possible new concept in the Management of Multiple Sclerosis Symptoms. Front Neurol. (2020) 11:152. doi: 10.3389/fneur.2020.00152, PMID: - DOI - PMC - PubMed
-
- Fernandez O, Costa-Frossard L, Martínez-Ginés ML, Montero P, Prieto-González JM, Ramió-Torrentà L. Integrated Management of Multiple Sclerosis Spasticity and Associated Symptoms Using the spasticity-plus syndrome concept: results of a structured specialists’ discussion using the Workmat® methodology. Front Neurol. (2021) 12:722801. doi: 10.3389/fneur.2021.722801, PMID: - DOI - PMC - PubMed
-
- Markovà J, Essner U, Akmaz B, Marinelli M, Trompke C, Lentschat A, et al. . Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci. (2019) 129:119–28. doi: 10.1080/00207454.2018.1481066, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

