Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection. Would Nanocarriers Complement Biofilm Disruption or Pave Its Road?
- PMID: 38708178
- PMCID: PMC11068056
- DOI: 10.2147/IJN.S445955
Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection. Would Nanocarriers Complement Biofilm Disruption or Pave Its Road?
Abstract
Introduction: Cystic fibrosis (CF) is associated with pulmonary Pseudomonas aeruginosa infections persistent to antibiotics.
Methods: To eradicate pseudomonal biofilms, solid lipid nanoparticles (SLNs) loaded with quorum-sensing-inhibitor (QSI, disrupting bacterial crosstalk), coated with chitosan (CS, improving internalization) and immobilized with alginate lyase (AL, destroying alginate biofilms) were developed.
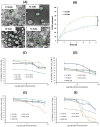
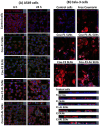
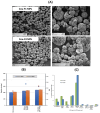
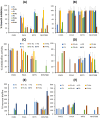
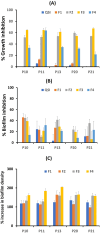
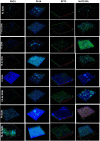
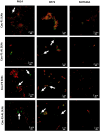
Results: SLNs (140-205 nm) showed prolonged release of QSI with no sign of acute toxicity to A549 and Calu-3 cells. The CS coating improved uptake, whereas immobilized-AL ensured >1.5-fold higher uptake and doubled SLN diffusion across the artificial biofilm sputum model. Respirable microparticles comprising SLNs in carbohydrate matrix elicited aerodynamic diameters MMAD (3.54, 2.48 µm) and fine-particle-fraction FPF (65, 48%) for anionic and cationic SLNs, respectively. The antimicrobial and/or antibiofilm activity of SLNs was explored in Pseudomonas aeruginosa reference mucoid/nonmucoid strains as well as clinical isolates. The full growth inhibition of planktonic bacteria was dependent on SLN type, concentration, growth medium, and strain. OD measurements and live/dead staining proved that anionic SLNs efficiently ceased biofilm formation and eradicated established biofilms, whereas cationic SLNs unexpectedly promoted biofilm progression. AL immobilization increased biofilm vulnerability; instead, CS coating increased biofilm formation confirmed by 3D-time lapse confocal imaging. Incubation of SLNs with mature biofilms of P. aeruginosa isolates increased biofilm density by an average of 1.5-fold. CLSM further confirmed the binding and uptake of the labeled SLNs in P. aeruginosa biofilms. Considerable uptake of CS-coated SLNs in non-mucoid strains could be observed presumably due to interaction of chitosan with LPS glycolipids in the outer cell membrane of P. aeruginosa.
Conclusion: The biofilm-destructive potential of QSI/SLNs/AL inhalation is promising for site-specific biofilm-targeted interventional CF therapy. Nevertheless, the intrinsic/extrinsic fundamentals of nanocarrier-biofilm interactions require further investigation.
Keywords: Pseudomonas aeruginosa; alginate lyase; chitosan; cystic fibrosis; quorum-sensing inhibitors; solid lipid nanoparticles.
© 2024 Nafee et al.
Conflict of interest statement
Martin Empting reports a patent WO2020007938A1 pending to Helmholtz Centre for Infection Research (HZI), a patent EP20150104 pending to Helmholtz Centre for Infection Research (HZI), and a patent WO2021136805A1 pending to Helmholtz Centre for Infection Research (HZI). The authors report no other conflicts of interest in this work.
Figures


References
-
- Cotton LA, Graham RJ, Lee RJ. The role of alginate in P. aeruginosa PAO1 biofilm structural resistance to gentamicin and ciprofloxacin. J Exp Microbiol Immunol. 2009;13:58–62.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

