Pregnancy-induced oxidative stress and inflammation are not associated with impaired maternal neuronal activity or memory function
- PMID: 38708544
- PMCID: PMC11381002
- DOI: 10.1152/ajpregu.00026.2024
Pregnancy-induced oxidative stress and inflammation are not associated with impaired maternal neuronal activity or memory function
Abstract
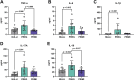
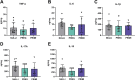
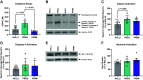
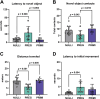
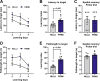
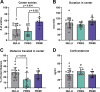
Pregnancy is associated with neural and behavioral plasticity, systemic inflammation, and oxidative stress, yet the impact of inflammation and oxidative stress on maternal neural and behavioral plasticity during pregnancy is unclear. We hypothesized that healthy pregnancy transiently reduces learning and memory and these deficits are associated with pregnancy-induced elevations in inflammation and oxidative stress. Cognitive performance was tested with novel object recognition (recollective memory), Morris water maze (spatial memory), and open field (anxiety-like) behavior tasks in female Sprague-Dawley rats of varying reproductive states [nonpregnant (nulliparous), pregnant (near term), and 1-2 mo after pregnancy (primiparous); n = 7 or 8/group]. Plasma and CA1 proinflammatory cytokines were measured with a MILLIPLEX magnetic bead assay. Plasma oxidative stress was measured via advanced oxidation protein products (AOPP) assay. CA1 markers of oxidative stress, neuronal activity, and apoptosis were quantified via Western blot analysis. Our results demonstrate that CA1 oxidative stress-associated markers were elevated in pregnant compared with nulliparous rats (P ≤ 0.017) but there were equivalent levels in pregnant and primiparous rats. In contrast, reproductive state did not impact CA1 inflammatory cytokines, neuronal activity, or apoptosis. Likewise, there was no effect of reproductive state on recollective or spatial memory. Even so, spatial learning was impaired (P ≤ 0.007) whereas anxiety-like behavior (P ≤ 0.034) was reduced in primiparous rats. Overall, our data suggest that maternal hippocampal CA1 is protected from systemic inflammation but vulnerable to peripartum oxidative stress. Peripartum oxidative stress elevations, such as in pregnancy complications, may contribute to peripartum neural and behavioral plasticity.NEW & NOTEWORTHY Healthy pregnancy is associated with elevated maternal systemic and brain oxidative stress. During postpregnancy, brain oxidative stress remains elevated whereas systemic oxidative stress is resolved. This sustained maternal brain oxidative stress is associated with learning impairments and decreased anxiety-like behavior during the postpregnancy period.
Keywords: anxiety-like behavior; cognition; inflammation; oxidative stress; parity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Update of
-
Pregnancy-associated oxidative stress and inflammation are not associated with impaired maternal neuronal activity or memory function.bioRxiv [Preprint]. 2024 Jan 27:2024.01.26.577461. doi: 10.1101/2024.01.26.577461. bioRxiv. 2024. Update in: Am J Physiol Regul Integr Comp Physiol. 2024 Jul 1;327(1):R35-R45. doi: 10.1152/ajpregu.00026.2024. PMID: 38328246 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

