Human bronchopulmonary disposition and plasma pharmacokinetics of oral bemnifosbuvir (AT-527), an experimental guanosine nucleotide prodrug for COVID-19
- PMID: 38708557
- PMCID: PMC11144486
- DOI: 10.1093/jac/dkae122
Human bronchopulmonary disposition and plasma pharmacokinetics of oral bemnifosbuvir (AT-527), an experimental guanosine nucleotide prodrug for COVID-19
Abstract
Background: Bemnifosbuvir (AT-527) is a novel oral guanosine nucleotide antiviral drug for the treatment of persons with COVID-19. Direct assessment of drug disposition in the lungs, via bronchoalveolar lavage, is necessary to ensure antiviral drug levels at the primary site of SARS-CoV-2 infection are achieved.
Objectives: This Phase 1 study in healthy subjects aimed to assess the bronchopulmonary pharmacokinetics, safety and tolerability of repeated doses of bemnifosbuvir.
Methods: A total of 24 subjects were assigned to receive bemnifosbuvir twice daily at doses of 275, 550 or 825 mg for up to 3.5 days.
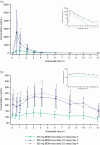
Results: AT-511, the free base of bemnifosbuvir, was largely eliminated from the plasma within 6 h post dose in all dosing groups. Antiviral drug levels of bemnifosbuvir were consistently achieved in the lungs with bemnifosbuvir 550 mg twice daily. The mean level of the guanosine nucleoside metabolite AT-273, the surrogate of the active triphosphate metabolite of the drug, measured in the epithelial lining fluid of the lungs was 0.62 µM at 4-5 h post dose. This exceeded the target in vitro 90% effective concentration (EC90) of 0.5 µM for antiviral drug exposure against SARS-CoV-2 replication in human airway epithelial cells. Bemnifosbuvir was well tolerated across all doses tested, and most treatment-emergent adverse events reported were mild in severity and resolved.
Conclusions: The favourable pharmacokinetics and safety profile of bemnifosbuvir demonstrates its potential as an oral antiviral treatment for COVID-19, with 550 mg bemnifosbuvir twice daily currently under further clinical evaluation in persons with COVID-19.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures



Similar articles
-
AT-527, a Double Prodrug of a Guanosine Nucleotide Analog, Is a Potent Inhibitor of SARS-CoV-2 In Vitro and a Promising Oral Antiviral for Treatment of COVID-19.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02479-20. doi: 10.1128/AAC.02479-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33558299 Free PMC article.
-
A Phase 2 Randomized Trial Evaluating the Antiviral Activity and Safety of the Direct-Acting Antiviral Bemnifosbuvir in Ambulatory Patients with Mild or Moderate COVID-19 (MOONSONG Study).Microbiol Spectr. 2023 Aug 17;11(4):e0007723. doi: 10.1128/spectrum.00077-23. Epub 2023 Jun 20. Microbiol Spectr. 2023. PMID: 37338393 Free PMC article. Clinical Trial.
-
Pharmacokinetics, Mass Balance, Safety, and Tolerability of Obeldesivir in Healthy Participants.Clin Pharmacol Ther. 2024 Nov;116(5):1231-1239. doi: 10.1002/cpt.3337. Epub 2024 Jun 28. Clin Pharmacol Ther. 2024. PMID: 38940465 Clinical Trial.
-
Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802.Clin Pharmacokinet. 1999 Dec;37(6):471-84. doi: 10.2165/00003088-199937060-00003. Clin Pharmacokinet. 1999. PMID: 10628898 Review.
-
Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations.J Antimicrob Chemother. 2010 Apr;65 Suppl 2(Suppl 2):ii5-ii10. doi: 10.1093/jac/dkq015. J Antimicrob Chemother. 2010. PMID: 20215135 Free PMC article. Review.
Cited by
-
Clinical Evaluation of Potential Interaction Between Bemnifosbuvir and Ruzasvir With an Assessment of Food Effect: Results of a Phase 1 Study in Healthy Participants.Clin Pharmacol Drug Dev. 2025 Jun;14(6):461-471. doi: 10.1002/cpdd.1540. Epub 2025 Apr 24. Clin Pharmacol Drug Dev. 2025. PMID: 40272375 Free PMC article. Clinical Trial.
References
-
- World Health Organization . Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

