Identification of a hemorrhagic determinant in Clostridioides difficile TcdA and Paeniclostridium sordellii TcsH
- PMID: 38709085
- PMCID: PMC11237598
- DOI: 10.1128/spectrum.00354-24
Identification of a hemorrhagic determinant in Clostridioides difficile TcdA and Paeniclostridium sordellii TcsH
Abstract
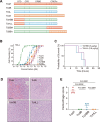
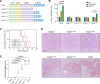
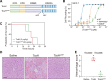
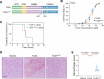
Paeniclostridium sordellii hemorrhagic toxin (TcsH) and Clostridioides difficile toxin A (TcdA) are two major members of the large clostridial toxin (LCT) family. These two toxins share ~87% similarity and are known to cause severe hemorrhagic pathology in animals. Yet, the pathogenesis of their hemorrhagic toxicity has been mysterious for decades. Here, we examined the liver injury after systemic exposure to different LCTs and found that only TcsH and TcdA induce overt hepatic hemorrhage. By investigating the chimeric and truncated toxins, we demonstrated that the enzymatic domain of TcsH alone is not sufficient to determine its potent hepatic hemorrhagic toxicity in mice. Likewise, the combined repetitive oligopeptide (CROP) domain of TcsH/TcdA alone also failed to explain their strong hemorrhagic activity in mice. Lastly, we showed that disrupting the first two short repeats of CROPs in TcsH and TcdA impaired hemorrhagic toxicity without causing overt changes in cytotoxicity and lethality. These findings lead to a deeper understanding of toxin-induced hemorrhage and the pathogenesis of LCTs and could be insightful in developing therapeutic avenues against clostridial infections.
Importance: Paeniclostridium sordellii and Clostridioides difficile infections often cause hemorrhage in the affected tissues and organs, which is mainly attributed to their hemorrhagic toxins, TcsH and TcdA. In this study, we demonstrate that TcsH and TcdA, but not other related toxins. including Clostridioides difficile toxin B and TcsL, induce severe hepatic hemorrhage in mice. We further determine that a small region in TcsH and TcdA is critical for the hemorrhagic toxicity but not cytotoxicity or lethality of these toxins. Based on these results, we propose that the hemorrhagic toxicity of TcsH and TcdA is due to an uncharacterized mechanism, such as the presence of an unknown receptor, and future studies to identify the interactive host factors are warranted.
Keywords: Clostridium difficile; Paeniclostridium sordellii; TcdA; TcsH; bacterial toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- 2023YFC2308403/MOST | National Key Research and Development Program of China (NKPs)
- 2024SSYS0036/Key Research and Development Program of Zhejiang Province (Key R&D plan of Zhejiang Province)
- LQ23C010001/MOST | NSFC | NSFC-Zhejiang Joint Fund | | Natural Science Foundation of Zhejiang Province (ZJNSF)
LinkOut - more resources
Full Text Sources
Medical

