Antibody-Drug Conjugate Sacituzumab Govitecan Enables a Sequential TOP1/PARP Inhibitor Therapy Strategy in Patients with Breast Cancer
- PMID: 38709212
- PMCID: PMC11247314
- DOI: 10.1158/1078-0432.CCR-24-0428
Antibody-Drug Conjugate Sacituzumab Govitecan Enables a Sequential TOP1/PARP Inhibitor Therapy Strategy in Patients with Breast Cancer
Abstract
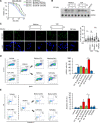
Purpose: The antibody-drug conjugate (ADC) sacituzumab govitecan (SG) comprises the topoisomerase 1 (TOP1) inhibitor (TOP1i) SN-38, coupled to a monoclonal antibody targeting trophoblast cell surface antigen 2 (TROP-2). Poly(ADP-ribose) polymerase (PARP) inhibition may synergize with TOP1i and SG, but previous studies combining systemic PARP and TOP1 inhibitors failed due to dose-limiting myelosuppression. Here, we assess the proof-of-mechanism and clinical feasibility for SG and talazoparib (TZP) employing an innovative sequential dosing schedule.
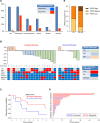
Patients and methods: In vitro models tested pharmacodynamic endpoints, and in a phase 1b clinical trial (NCT04039230), 30 patients with metastatic triple-negative breast cancer (mTNBC) received SG and TZP in a concurrent (N = 7) or sequential (N = 23) schedule. Outcome measures included safety, tolerability, preliminary efficacy, and establishment of a recommended phase 2 dose.
Results: We hypothesized that tumor-selective delivery of TOP1i via SG would reduce nontumor toxicity and create a temporal window, enabling sequential dosing of SG and PARP inhibition. In vitro, sequential SG followed by TZP delayed TOP1 cleavage complex clearance, increased DNA damage, and promoted apoptosis. In the clinical trial, sequential SG/TZP successfully met primary objectives and demonstrated median progression-free survival (PFS) of 7.6 months without dose-limiting toxicities (DLT), while concurrent dosing yielded 2.3 months PFS and multiple DLTs including severe myelosuppression.
Conclusions: While SG dosed concurrently with TZP is not tolerated clinically due to an insufficient therapeutic window, sequential dosing of SG followed by TZP proved a viable strategy. These findings support further clinical development of the combination and suggest that ADC-based therapy may facilitate novel, mechanism-based dosing strategies.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Bardia reports grants and personal fees from Pfizer, Novartis, Genentech, Merck, Menarini, Gilead, Sanofi, Daiichi Pharma/Astra Zeneca, and from Eli Lilly during the conduct of the study; in addition, A. Bardia has a patent for ADC and PARP combination pending. L. Spring reports acting as consultant and as member of the advisory board for Novartis, Daiichi Pharma, AstraZeneca, Eli Lilly, Precede, and Seagen as well as providing institutional research support for Merck, Genentech, Gilead, and Eli Lilly. A. Partridge reports other support from Novartis and from Wolters Kluwer outside the submitted work. D. Juric reports grants and personal fees from Novartis, Genentech, Syros, Eisai, Pfizer, and Takeda; personal fees from Vibliome, PIC Therapeutics, Mapkure, and Relay Therapeutics; and grants from Amgen, InventisBio, Arvinas, Blueprint, AstraZeneca, and from Ribon Therapeutics outside the submitted work. J. Peppercorn reports personal fees from GSK outside the submitted work. H. Parsons reports personal fees from Guardant Health, AstraZeneca, Daiichi-Sankyo, Sermonix, Caris Life Sciences, and from Illumina outside the submitted work. S.A. Wander reports personal fees from Foundation Medicine, Eli Lilly, Novartis, AstraZeneca, Puma Biotechnology, Genentech, Pfizer, Hologic, and Biovica, as well as other support from 2ndMD, Eli Lilly, Guardant Health, and from Genentech, Pfizer, Regor Therapeutics, Sermonix, and Nuvation Bio outside the submitted work. V. Attaya reports personal fees from Olema Oncology outside the submitted work. A. Nagayama reports personal fees from Chugai Pharmaceutical, Pfizer, Daiichi Sankyo, and from Eli Lilly Japan K.K. outside the submitted work. S.M. Tolaney reports grants from Gilead during the conduct of the study; grants and personal fees from Novartis, Pfizer (SeaGen), Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, and Bristol Myers Squibb; personal fees from Sanofi, CytomX Therapeutics, Daiichi Sankyo, Gilead, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, Sumitovant Biopharma, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Bio, Bayer, Incyte Corp., Jazz Pharmaceuticals, Natera, Tango Therapeutics, Systimmune, eFFECTOR, Hengrui USA, Cullinan Oncology, Circle Pharma, and Arvinas; and grants from Exelixis, NanoString Technologies, Seattle Genetics, and from OncoPep outside the submitted work. L.W. Ellisen reports other support from Gilead during the conduct of the study; personal fees from Mersana, Inc., Astra Zeneca, and from Kisoji Biotechnology outside the submitted work; in addition, L.W. Ellisen has a patent for 63/329,505 pending to Ellisen/Bardia and research funding from Sanofi. No disclosures were reported by the other authors.
Figures
References
-
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. ; ASCENT Clinical Trial Investigators . Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 2021;384:1529–41. - PubMed
-
- Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 2019;380:741–51. - PubMed
-
- Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 2021;32:746–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

