Intraoperative haemoadsorption for antithrombotic drug removal during cardiac surgery: initial report of the international safe and timely antithrombotic removal (STAR) registry
- PMID: 38709456
- PMCID: PMC11315775
- DOI: 10.1007/s11239-024-02996-x
Intraoperative haemoadsorption for antithrombotic drug removal during cardiac surgery: initial report of the international safe and timely antithrombotic removal (STAR) registry
Abstract
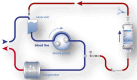
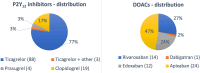
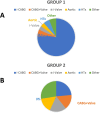
Intraoperative antithrombotic drug removal by haemoadsorption is a novel strategy to reduce perioperative bleeding in patients on antithrombotic drugs undergoing cardiac surgery. The international STAR registry reports real-world clinical outcomes associated with this application. All patients underwent cardiac surgery before completing the recommended washout period. The haemoadsorption device was incorporated into the cardiopulmonary bypass (CPB) circuit. Patients on P2Y12 inhibitors comprised group 1, and patients on direct-acting oral anticoagulants (DOAC) group 2. Outcome measurements included bleeding events according to standardised definitions and 24-hour chest-tube-drainage (CTD). 165 patients were included from 8 institutions in Austria, Germany, Sweden, and the UK. Group 1 included 114 patients (62.9 ± 11.6years, 81% male) operated at a mean time of 33.2 h from the last P2Y12 inhibitor dose with a mean CPB duration of 117.1 ± 62.0 min. Group 2 included 51 patients (68.4 ± 9.4years, 53% male), operated at a mean time of 44.6 h after the last DOAC dose, with a CPB duration of 128.6 ± 48.4 min. In Group 1, 15 patients experienced a BARC-4 bleeding event (13%), including 3 reoperations (2.6%). The mean 24-hour CTD was 651 ± 407mL. In Group 2, 8 patients experienced a BARC-4 bleeding event (16%) including 4 reoperations (7.8%). The mean CTD was 675 ± 363mL. This initial report of the ongoing STAR registry shows that the intraoperative use of a haemoadsorption device is simple and safe, and may potentially mitigate the expected high bleeding risk of patients on antithrombotic drugs undergoing cardiac surgery before completion of the recommended washout period.Clinical registration number: ClinicalTrials.gov identifier: NCT05077124.
Keywords: Antithrombotic removal; Cardiac surgery; CytoSorb; DOAC; Haemoadsorption; Ticagrelor.
© 2024. The Author(s).
Conflict of interest statement
Michael Schmoeckel, Matthias Thielmann, Kambiz Hassan, Stephan Geidel, Sandra Lindstedt, received speaker honoraria and travel fees. Marijana Matejic-Spasic, Daniel Wendt are full-time employees of CytoSorbents Europe GmbH, Berlin, Germany, Efthymios Deliargyris is a full-time employee of CytoSorbents Inc., Princeton, NJ, USA. Robert Storey reports institutional research grants/support from Cytosorbents and AstraZeneca; and personal fees from Alfasigma, AstraZeneca, Boehringer Ingelheim/Lilly, Chiesi, Cytosorbents, Daiichi Sankyo, Idorsia, Novartis, Novo Nordisk, Pfizer, PhaseBio and Tabuk.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

