Whole-Body Hypothermia vs Targeted Normothermia for Neonates With Mild Encephalopathy: A Multicenter Pilot Randomized Clinical Trial
- PMID: 38709535
- PMCID: PMC11074808
- DOI: 10.1001/jamanetworkopen.2024.9119
Whole-Body Hypothermia vs Targeted Normothermia for Neonates With Mild Encephalopathy: A Multicenter Pilot Randomized Clinical Trial
Abstract
Importance: Although whole-body hypothermia is widely used after mild neonatal hypoxic-ischemic encephalopathy (HIE), safety and efficacy have not been evaluated in randomized clinical trials (RCTs), to our knowledge.
Objective: To examine the effect of 48 and 72 hours of whole-body hypothermia after mild HIE on cerebral magnetic resonance (MR) biomarkers.
Design, setting, and participants: This open-label, 3-arm RCT was conducted between October 31, 2019, and April 28, 2023, with masked outcome analysis. Participants were neonates at 6 tertiary neonatal intensive care units in the UK and Italy born at or after 36 weeks' gestation with severe birth acidosis, requiring continued resuscitation, or with an Apgar score less than 6 at 10 minutes after birth and with evidence of mild HIE on modified Sarnat staging. Statistical analysis was per intention to treat.
Interventions: Random allocation to 1 of 3 groups (1:1:1) based on age: neonates younger than 6 hours were randomized to normothermia or 72-hour hypothermia (33.5 °C), and those 6 hours or older and already receiving whole-body hypothermia were randomized to rewarming after 48 or 72 hours of hypothermia.
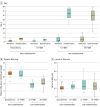
Main outcomes and measures: Thalamic N-acetyl aspartate (NAA) concentration (mmol/kg wet weight), assessed by cerebral MR imaging and thalamic spectroscopy between 4 and 7 days after birth using harmonized sequences.
Results: Of 225 eligible neonates, 101 were recruited (54 males [53.5%]); 48 (47.5%) were younger than 6 hours and 53 (52.5%) were 6 hours or older at randomization. Mean (SD) gestational age and birth weight were 39.5 (1.1) weeks and 3378 (380) grams in the normothermia group (n = 34), 38.7 (0.5) weeks and 3017 (338) grams in the 48-hour hypothermia group (n = 31), and 39.0 (1.1) weeks and 3293 (252) grams in the 72-hour hypothermia group (n = 36). More neonates in the 48-hour (14 of 31 [45.2%]) and 72-hour (13 of 36 [36.1%]) groups required intubation at birth than in the normothermic group (3 of 34 [8.8%]). Ninety-nine neonates (98.0%) had MR imaging data and 87 (86.1%), NAA data. Injury scores on conventional MR biomarkers were similar across groups. The mean (SD) NAA level in the normothermia group was 10.98 (0.92) mmol/kg wet weight vs 8.36 (1.23) mmol/kg wet weight (mean difference [MD], -2.62 [95% CI, -3.34 to -1.89] mmol/kg wet weight) in the 48-hour and 9.02 (1.79) mmol/kg wet weight (MD, -1.96 [95% CI, -2.66 to -1.26] mmol/kg wet weight) in the 72-hour hypothermia group. Seizures occurred beyond 6 hours after birth in 4 neonates: 1 (2.9%) in the normothermia group, 1 (3.2%) in the 48-hour hypothermia group, and 2 (5.6%) in the 72-hour hypothermia group.
Conclusions and relevance: In this pilot RCT, whole-body hypothermia did not improve cerebral MR biomarkers after mild HIE, although neonates in the hypothermia groups were sicker at baseline. Safety and efficacy of whole-body hypothermia should be evaluated in RCTs.
Trial registration: ClinicalTrials.gov Identifier: NCT03409770.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

