Enantioselective transformation of phytoplankton-derived dihydroxypropanesulfonate by marine bacteria
- PMID: 38709871
- PMCID: PMC11131964
- DOI: 10.1093/ismejo/wrae084
Enantioselective transformation of phytoplankton-derived dihydroxypropanesulfonate by marine bacteria
Abstract
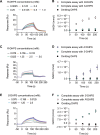
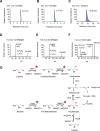
Chirality, a fundamental property of matter, is often overlooked in the studies of marine organic matter cycles. Dihydroxypropanesulfonate (DHPS), a globally abundant organosulfur compound, serves as an ecologically important currency for nutrient and energy transfer from phytoplankton to bacteria in the ocean. However, the chirality of DHPS in nature and its transformation remain unclear. Here, we developed a novel approach using chiral phosphorus-reagent labeling to separate DHPS enantiomers. Our findings demonstrated that at least one enantiomer of DHPS is present in marine diatoms and coccolithophores, and that both enantiomers are widespread in marine environments. A novel chiral-selective DHPS catabolic pathway was identified in marine Roseobacteraceae strains, where HpsO and HpsP dehydrogenases at the gateway to DHPS catabolism act specifically on R-DHPS and S-DHPS, respectively. R-DHPS is also a substrate for the dehydrogenase HpsN. All three dehydrogenases generate stable hydrogen bonds between the chirality-center hydroxyls of DHPS and highly conserved residues, and HpsP also form coordinate-covalent bonds between the chirality-center hydroxyls and Zn2+, which determines the mechanistic basis of strict stereoselectivity. We further illustrated the role of enzymatic promiscuity in the evolution of DHPS metabolism in Roseobacteraceae and SAR11. This study provides the first evidence of chirality's involvement in phytoplankton-bacteria metabolic currencies, opening a new avenue for understanding the ocean organosulfur cycle.
Keywords: Roseobacteraceae; bacteria; chirality; ocean; organosulfur cycle; phytoplankton.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Devínsky F. Chirality and the origin of life. Symmetry 2021;13:2277. 10.3390/sym13122277. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

