A high-fat diet promotes cancer progression by inducing gut microbiota-mediated leucine production and PMN-MDSC differentiation
- PMID: 38709933
- PMCID: PMC11098111
- DOI: 10.1073/pnas.2306776121
A high-fat diet promotes cancer progression by inducing gut microbiota-mediated leucine production and PMN-MDSC differentiation
Abstract
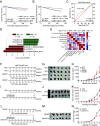
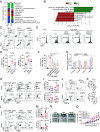
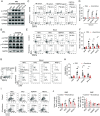
A high-fat diet (HFD) is a high-risk factor for the malignant progression of cancers through the disruption of the intestinal microbiota. However, the role of the HFD-related gut microbiota in cancer development remains unclear. This study found that obesity and obesity-related gut microbiota were associated with poor prognosis and advanced clinicopathological status in female patients with breast cancer. To investigate the impact of HFD-associated gut microbiota on cancer progression, we established various models, including HFD feeding, fecal microbiota transplantation, antibiotic feeding, and bacterial gavage, in tumor-bearing mice. HFD-related microbiota promotes cancer progression by generating polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs). Mechanistically, the HFD microbiota released abundant leucine, which activated the mTORC1 signaling pathway in myeloid progenitors for PMN-MDSC differentiation. Clinically, the elevated leucine level in the peripheral blood induced by the HFD microbiota was correlated with abundant tumoral PMN-MDSC infiltration and poor clinical outcomes in female patients with breast cancer. These findings revealed that the "gut-bone marrow-tumor" axis is involved in HFD-mediated cancer progression and opens a broad avenue for anticancer therapeutic strategies by targeting the aberrant metabolism of the gut microbiota.
Keywords: breast cancer; gut microbiota; high-fat diet; myeloid-derived suppressor cells.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R., Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 (2010). - PubMed
-
- Calle E. E., Kaaks R., Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004). - PubMed
-
- van Kruijsdijk R. C., van der Wall E., Visseren F. L., Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomarkers Prev. 18, 2569–2578 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2019BT02Y198/Program for Guangdong Introducing Innovative and Enterpreneurial Teams
- 82330056/Natural Science Foundation of China
- 81730077/National Natural Science Foundation of China
- 92159303/National Natural Science Foundation of China
- 81930081/National Natural Science Foundation of China
- 82025026/National Natural Science Foundation of China
- 2022A1515110069/GDSTC | Basic and Applied Basic Research Foundation of Guangdong Province ()
- 2023B1212060013/Guangdong Science and Technology Department
- 20212200003/Bureau of Science and Technology of Guangzhou
- E2018096/Guang Dong Cheung Kong philanthropy Foundation
LinkOut - more resources
Full Text Sources
Medical

