Benfotiamine improves dystrophic pathology and exercise capacity in mdx mice by reducing inflammation and fibrosis
- PMID: 38710523
- PMCID: PMC11262745
- DOI: 10.1093/hmg/ddae066
Benfotiamine improves dystrophic pathology and exercise capacity in mdx mice by reducing inflammation and fibrosis
Abstract
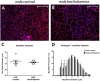
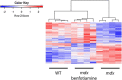
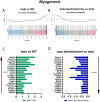
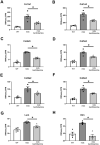
Duchenne Muscular Dystrophy (DMD) is a progressive and fatal neuromuscular disease. Cycles of myofibre degeneration and regeneration are hallmarks of the disease where immune cells infiltrate to repair damaged skeletal muscle. Benfotiamine is a lipid soluble precursor to thiamine, shown clinically to reduce inflammation in diabetic related complications. We assessed whether benfotiamine administration could reduce inflammation related dystrophic pathology. Benfotiamine (10 mg/kg/day) was fed to male mdx mice (n = 7) for 15 weeks from 4 weeks of age. Treated mice had an increased growth weight (5-7 weeks) and myofibre size at treatment completion. Markers of dystrophic pathology (area of damaged necrotic tissue, central nuclei) were reduced in benfotiamine mdx quadriceps. Grip strength was increased and improved exercise capacity was found in mdx treated with benfotiamine for 12 weeks, before being placed into individual cages and allowed access to an exercise wheel for 3 weeks. Global gene expression profiling (RNAseq) in the gastrocnemius revealed benfotiamine regulated signalling pathways relevant to dystrophic pathology (Inflammatory Response, Myogenesis) and fibrotic gene markers (Col1a1, Col1a2, Col4a5, Col5a2, Col6a2, Col6a2, Col6a3, Lum) towards wildtype levels. In addition, we observed a reduction in gene expression of inflammatory gene markers in the quadriceps (Emr1, Cd163, Cd4, Cd8, Ifng). Overall, these data suggest that benfotiamine reduces dystrophic pathology by acting on inflammatory and fibrotic gene markers and signalling pathways. Given benfotiamine's excellent safety profile and current clinical use, it could be used in combination with glucocorticoids to treat DMD patients.
Keywords: Duchenne; benfotiamine; inflammation; muscular dystrophy.
© The Author(s) 2024. Published by Oxford University Press.
Figures







References
-
- Mendell JR, Shilling C, Leslie ND. et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. - PubMed
-
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928. - PubMed
-
- Campbell KP, Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature 1989;338:259–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

