A Japanese herbal medicine (kampo), hochuekkito (TJ-41), has anti-inflammatory effects on the chronic obstructive pulmonary disease mouse model
- PMID: 38710754
- PMCID: PMC11074295
- DOI: 10.1038/s41598-024-60646-x
A Japanese herbal medicine (kampo), hochuekkito (TJ-41), has anti-inflammatory effects on the chronic obstructive pulmonary disease mouse model
Abstract
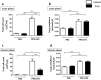
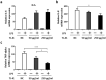
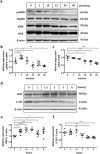
Chronic obstructive pulmonary disease (COPD) is a progressive disease that is characterized by chronic airway inflammation. A Japanese herbal medicine, hochuekkito (TJ-41), is prominently used for chronic inflammatory diseases in Japan. This study aimed to analyze the anti-inflammatory effect of TJ-41 in vivo and its underlying mechanisms. We created a COPD mouse model using intratracheal administration of porcine pancreatic elastase and lipopolysaccharide (LPS) and analyzed them with and without TJ-41 administration. A TJ-41-containing diet reduced inflammatory cell infiltration of the lungs in the acute and chronic phases and body weight loss in the acute phase. In vitro experiments revealed that TJ-41 treatment suppressed the LPS-induced inflammatory cytokines in BEAS-2B cells. Furthermore, TJ-41 administration activated the AMP-activated protein kinase (AMPK) pathway and inhibited the mechanistic target of the rapamycin (mTOR) pathway, both in cellular and mouse experiments. We concluded that TJ-41 administration reduced airway inflammation in the COPD mouse model, which might be regulated by the activated AMPK pathway, and inhibited the mTOR pathway.
© 2024. The Author(s).
Conflict of interest statement
AM, HI, and HT received research funding from Tsumura & Co. (Tokyo, Japan). TJ has received financial contributions from Tsumura & Co. because he has academic affiliations with the Department of Health Services Research, Graduate School of Medicine, and The University of Tokyo supported by Tsumura & Co. The authors declare no other competing interests in this work.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

