Targeting pathological cells with senolytic drugs reduces seizures in neurodevelopmental mTOR-related epilepsy
- PMID: 38710875
- PMCID: PMC11156583
- DOI: 10.1038/s41593-024-01634-2
Targeting pathological cells with senolytic drugs reduces seizures in neurodevelopmental mTOR-related epilepsy
Abstract
Cortical malformations such as focal cortical dysplasia type II (FCDII) are associated with pediatric drug-resistant epilepsy that necessitates neurosurgery. FCDII results from somatic mosaicism due to post-zygotic mutations in genes of the PI3K-AKT-mTOR pathway, which produce a subset of dysmorphic cells clustered within healthy brain tissue. Here we show a correlation between epileptiform activity in acute cortical slices obtained from human surgical FCDII brain tissues and the density of dysmorphic neurons. We uncovered multiple signatures of cellular senescence in these pathological cells, including p53/p16 expression, SASP expression and senescence-associated β-galactosidase activity. We also show that administration of senolytic drugs (dasatinib/quercetin) decreases the load of senescent cells and reduces seizure frequency in an MtorS2215F FCDII preclinical mouse model, providing proof of concept that senotherapy may be a useful approach to control seizures. These findings pave the way for therapeutic strategies selectively targeting mutated senescent cells in FCDII brain tissue.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

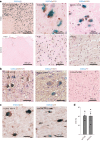
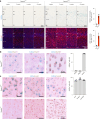
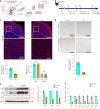
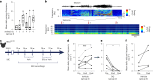
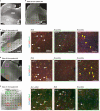





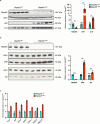
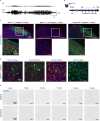
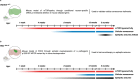
Comment in
-
Reducing seizures with senolytic drugs.Nat Rev Drug Discov. 2024 Jul;23(7):499. doi: 10.1038/d41573-024-00092-6. Nat Rev Drug Discov. 2024. PMID: 38822116 No abstract available.
References
-
- Guerrini R, Barba C. Focal cortical dysplasia: an update on diagnosis and treatment. Exp. Rev. Neurother. 2021;21:1213–1224. - PubMed
-
- Blumcke I, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N. Engl. J. Med. 2017;377:1648–1656. - PubMed
-
- Lamparello P, et al. Developmental lineage of cell types in cortical dysplasia with balloon cells. Brain. 2007;130:2267–2276. - PubMed
-
- Bizzotto S, Walsh CA. Genetic mosaicism in the human brain: from lineage tracing to neuropsychiatric disorders. Nat. Rev. Neurosci. 2022;23:275–286. - PubMed
-
- Blumcke I, et al. Neocortical development and epilepsy: insights from focal cortical dysplasia and brain tumours. Lancet Neurol. 2021;20:943–955. - PubMed
MeSH terms
Substances
Grants and funding
- 682345/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 101113154/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- ANR-11-IDEX-0004-02/Agence Nationale de la Recherche (French National Research Agency)
- ANR-10-IAIHU-06/Agence Nationale de la Recherche (French National Research Agency)
- ECO20160736027/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

