Development of a scalable recombinant system for cyclic beta-1,2-glucans production
- PMID: 38711033
- PMCID: PMC11071196
- DOI: 10.1186/s12934-024-02407-z
Development of a scalable recombinant system for cyclic beta-1,2-glucans production
Abstract
Background: Cyclic β-1,2-glucans (CβG) are bacterial cyclic homopolysaccharides with interesting biotechnological applications. These ring-shaped molecules have a hydrophilic surface that confers high solubility and a hydrophobic cavity able to include poorly soluble molecules. Several studies demonstrate that CβG and many derivatives can be applied in drug solubilization and stabilization, enantiomer separation, catalysis, synthesis of nanomaterials and even as immunomodulators, suggesting these molecules have great potential for their industrial and commercial exploitation. Nowadays, there is no method to produce CβG by chemical synthesis and bacteria that synthesize them are slow-growing or even pathogenic, which makes the scaling up of the process difficult and expensive. Therefore, scalable production and purification methods are needed to afford the demand and expand the repertoire of applications of CβG.
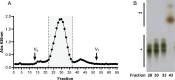
Results: We present the production of CβG in specially designed E. coli strains by means of the deletion of intrinsic polysaccharide biosynthetic genes and the heterologous expression of enzymes involved in CβG synthesis, transport and succinilation. These strains produce different types of CβG: unsubstituted CβG, anionic CβG and CβG of high size. Unsubstituted CβG with a degree of polymerization of 17 to 24 glucoses were produced and secreted to the culture medium by one of the strains. Through high cell density culture (HCDC) of that strain we were able to produce 4,5 g of pure unsubstituted CβG /L in culture medium within 48 h culture.
Conclusions: We have developed a new recombinant bacterial system for the synthesis of cyclic β-1,2-glucans, expanding the use of bacteria as a platform for the production of new polysaccharides with biotechnological applications. This new approach allowed us to produce CβG in E. coli with high yields and the highest volumetric productivity reported to date. We expect this new highly scalable system facilitates CβG availability for further research and the widespread use of these promising molecules across many application fields.
Keywords: Cyclic β-1,2-glucans; Cyclodextrin; Cyclosophoraoses; Drug solubilization; Nanomaterial; Oligosaccharides.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Venkatachalam G, Arumugam S, Doble M. Industrial production and applications of α/β linear and branched glucans. Indian Chem Eng. 2021;63(5):533–47. doi: 10.1080/00194506.2020.1798820. - DOI
-
- Yates LE, Mills DC, DeLisa MP. Bacterial glycoengineering as a Biosynthetic Route to customized glycomolecules. In: Rapp E, Reichl U, editors. Advances in Glycobiotechnology. Cham: Springer International Publishing; 2018. pp. 167–200. - PubMed
MeSH terms
Substances
Grants and funding
- PICT-2017-2084/Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina
- PICT-2017-2084/Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina
- PICT-2017-2084/Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina
- PICT-2017-2084/Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina
- PICT-2017-2084/Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina
LinkOut - more resources
Full Text Sources

