D-mannose alleviates intervertebral disc degeneration through glutamine metabolism
- PMID: 38711073
- PMCID: PMC11071241
- DOI: 10.1186/s40779-024-00529-4
D-mannose alleviates intervertebral disc degeneration through glutamine metabolism
Abstract
Background: Intervertebral disc degeneration (IVDD) is a multifaceted condition characterized by heterogeneity, wherein the balance between catabolism and anabolism in the extracellular matrix of nucleus pulposus (NP) cells plays a central role. Presently, the available treatments primarily focus on relieving symptoms associated with IVDD without offering an effective cure targeting its underlying pathophysiological processes. D-mannose (referred to as mannose) has demonstrated anti-catabolic properties in various diseases. Nevertheless, its therapeutic potential in IVDD has yet to be explored.
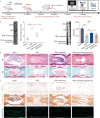
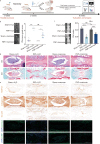
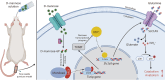
Methods: The study began with optimizing the mannose concentration for restoring NP cells. Transcriptomic analyses were employed to identify the mediators influenced by mannose, with the thioredoxin-interacting protein (Txnip) gene showing the most significant differences. Subsequently, small interfering RNA (siRNA) technology was used to demonstrate that Txnip is the key gene through which mannose exerts its effects. Techniques such as colocalization analysis, molecular docking, and overexpression assays further confirmed the direct regulatory relationship between mannose and TXNIP. To elucidate the mechanism of action of mannose, metabolomics techniques were employed to pinpoint glutamine as a core metabolite affected by mannose. Next, various methods, including integrated omics data and the Gene Expression Omnibus (GEO) database, were used to validate the one-way pathway through which TXNIP regulates glutamine. Finally, the therapeutic effect of mannose on IVDD was validated, elucidating the mechanistic role of TXNIP in glutamine metabolism in both intradiscal and orally treated rats.
Results: In both in vivo and in vitro experiments, it was discovered that mannose has potent efficacy in alleviating IVDD by inhibiting catabolism. From a mechanistic standpoint, it was shown that mannose exerts its anti-catabolic effects by directly targeting the transcription factor max-like protein X-interacting protein (MondoA), resulting in the upregulation of TXNIP. This upregulation, in turn, inhibits glutamine metabolism, ultimately accomplishing its anti-catabolic effects by suppressing the mitogen-activated protein kinase (MAPK) pathway. More importantly, in vivo experiments have further demonstrated that compared with intradiscal injections, oral administration of mannose at safe concentrations can achieve effective therapeutic outcomes.
Conclusions: In summary, through integrated multiomics analysis, including both in vivo and in vitro experiments, this study demonstrated that mannose primarily exerts its anti-catabolic effects on IVDD through the TXNIP-glutamine axis. These findings provide strong evidence supporting the potential of the use of mannose in clinical applications for alleviating IVDD. Compared to existing clinically invasive or pain-relieving therapies for IVDD, the oral administration of mannose has characteristics that are more advantageous for clinical IVDD treatment.
Keywords: D-mannose; Glutamine; Intervertebral disc degeneration; Thioredoxin-interacting protein (TXNIP).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Figures






References
-
- Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi: 10.1016/S0140-6736(20)32340-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

