PI3K/mTOR inhibition induces tumour microenvironment remodelling and sensitises pS6high uterine leiomyosarcoma to PD-1 blockade
- PMID: 38711203
- PMCID: PMC11074386
- DOI: 10.1002/ctm2.1655
PI3K/mTOR inhibition induces tumour microenvironment remodelling and sensitises pS6high uterine leiomyosarcoma to PD-1 blockade
Abstract
Background: Uterine leiomyosarcomas (uLMS) are aggressive tumours with poor prognosis and limited treatment options. Although immune checkpoint blockade (ICB) has proven effective in some 'challenging-to-treat' cancers, clinical trials showed that uLMS do not respond to ICB. Emerging evidence suggests that aberrant PI3K/mTOR signalling can drive resistance to ICB. We therefore explored the relevance of the PI3K/mTOR pathway for ICB treatment in uLMS and explored pharmacological inhibition of this pathway to sensitise these tumours to ICB.
Methods: We performed an integrated multiomics analysis based on TCGA data to explore the correlation between PI3K/mTOR dysregulation and immune infiltration in 101 LMS. We assessed response to PI3K/mTOR inhibitors in immunodeficient and humanized uLMS patient-derived xenografts (PDXs) by evaluating tumour microenvironment modulation using multiplex immunofluorescence. We explored response to single-agent and a combination of PI3K/mTOR inhibitors with PD-1 blockade in humanized uLMS PDXs. We mapped intratumoural dynamics using single-cell RNA/TCR sequencing of serially collected biopsies.
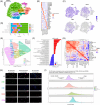
Results: PI3K/mTOR over-activation (pS6high) associated with lymphocyte depletion and wound healing immune landscapes in (u)LMS, suggesting it contributes to immune evasion. In contrast, PI3K/mTOR inhibition induced profound tumour microenvironment remodelling in an ICB-resistant humanized uLMS PDX model, fostering adaptive anti-tumour immune responses. Indeed, PI3K/mTOR inhibition induced macrophage repolarisation towards an anti-tumourigenic phenotype and increased antigen presentation on dendritic and tumour cells, but also promoted infiltration of PD-1+ T cells displaying an exhausted phenotype. When combined with anti-PD-1, PI3K/mTOR inhibition led to partial or complete tumour responses, whereas no response to single-agent anti-PD-1 was observed. Combination therapy reinvigorated exhausted T cells and induced clonal hyper-expansion of a cytotoxic CD8+ T-cell population supported by a CD4+ Th1 niche.
Conclusions: Our findings indicate that aberrant PI3K/mTOR pathway activation contributes to immune escape in uLMS and provides a rationale for combining PI3K/mTOR inhibition with ICB for the treatment of this patient population.
Keywords: PI3K/mTOR inhibitors; anti‐PD‐1 therapy; humanized patient‐derived xenograft models; immune‐modulation; resistance; uterine leiomyosarcoma.
© 2024 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures





References
-
- Amant F, Coosemans An, Debiec‐Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188‐1198. - PubMed
-
- Fares CM, Van Allen EM, Drake CG, Allison JP, Hu‐Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. 2019;39:147‐164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
