Managing potential adverse events during treatment with enfortumab vedotin + pembrolizumab in patients with advanced urothelial cancer
- PMID: 38711854
- PMCID: PMC11071165
- DOI: 10.3389/fonc.2024.1326715
Managing potential adverse events during treatment with enfortumab vedotin + pembrolizumab in patients with advanced urothelial cancer
Abstract
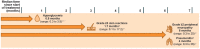
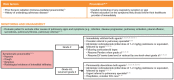
Cisplatin-based chemotherapy has been the standard of care for patients with locally advanced or metastatic urothelial cancer (la/mUC). Enfortumab vedotin, an antibody-drug conjugate directed to Nectin-4, and pembrolizumab, an immune checkpoint inhibitor, are two therapies that have individually provided a survival benefit in patients with la/mUC. The combination regimen of enfortumab vedotin plus pembrolizumab was evaluated in EV-302 (KEYNOTE-A39; NCT0422385), a phase 3 study that showed statistically significant and clinically meaningful improvement in overall survival, progression-free survival, and a key secondary endpoint of overall response rate versus chemotherapy. Based on these results and those from the EV-103 (KEYNOTE-869; NCT03288545) Dose Escalation cohort, Cohort A, and Cohort K, enfortumab vedotin plus pembrolizumab was granted approval from the US Food and Drug Administration for the treatment of adults with la/mUC. While guidelines and recommendations for the management of adverse events (AEs) have been developed for immune checkpoint inhibitor monotherapy and enfortumab vedotin monotherapy, additional guidance is needed for managing AEs that occur with enfortumab vedotin plus pembrolizumab. As monotherapies, enfortumab vedotin and pembrolizumab are both associated with some of the AEs observed with the combination, such as skin reactions, pneumonitis, and diarrhea, which may confound the attribution of the AE to a specific agent and thereby complicate clinical management. In this manuscript, we aim to provide recommendations for best practice for patient care and the management of AEs of clinical interest for patients with la/mUC receiving enfortumab vedotin plus pembrolizumab, including skin reactions, peripheral neuropathy, hyperglycemia, and pneumonitis. These recommendations were developed based on published guidelines, expert opinions, and the clinical experience of the authors, which include oncologist, advanced practice provider, nursing, and pharmacy perspectives. In addition, guidance on patient education and communication is provided. With vigilant monitoring, early detection, and prompt intervention of treatment-emergent AEs based on recommended approaches described herein, it is the authors' experience that most AEs can be managed with supportive therapy and dose modification/interruptions, allowing patients to continue treatment.
Keywords: adverse events; anticancer therapy; enfortumab vedotin; immune-related adverse events; irAE; pembrolizumab; urothelial cancer.
Copyright © 2024 Brower, McCoy, Ahmad, Eitman, Bowman, Rembisz and Milowsky.
Conflict of interest statement
The authors declare the following competing interests: BB: Consulting/Advisory: Seagen; AM: Consulting/Advisory: Seagen; CE: Consulting/Advisory: Astellas; Speaker's Bureau: Pfizer; IB: Consulting/Advisory: AstraZeneca, Aveo, Janssen; Speakers Bureau: Natera, Caris Life Sciences; JR: Consulting/Advisory: Astellas; Honoraria: Gilead Sciences; Other: Moffitt Cancer Center; MM: Stock and Other Ownership Interests: Pfizer, Merck, Gilead Sciences; Consulting or Advisory Role: Loxo/Lilly; Research Funding: Merck Inst, Roche/Genentech Inst, Bristol Myers Squibb Inst, Mirati Therapeutics Inst, Incyte Inst, Seagen Inst, G1 Therapeutics Inst, Alliance Foundation Trials Inst, Alliance for Clinical Trials in Oncology Inst, Clovis Oncology Inst, Arvinas Inst, ALX Oncology Inst, Loxo Inst, Hoosier Cancer Research Network Inst; Other Relationship: Elsevier, Medscape. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This study received funding from Astellas Pharma US; Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; and Seagen Inc. The funder had the following involvement with the study: study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript.
Figures



References
-
- Shah MV, McGovern A, Hepp Z. PCN108 - targeted literature review of the burden of illness in urothelial carcinoma. Value Health. (2018) 21:S32–3. doi: 10.1016/j.jval.2018.09.191 - DOI
-
- National Cancer Institute . SEER Cancer Stat Facts: Bladder Cancer (2024). Available online at: https://seer.cancer.gov/statfacts/html/urinb.html (Accessed January 17, 2024).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

